Study determines DHA and ARA essential to meet dietary requirements

The California yellowtail (Seriola dorsalis, formerly S. lalandi), an important food and sportfish, is an important commercial aquaculture candidate and there is much interest to develop commercial, offshore production operations in the western United States and Mexico. But to achieve this aquaculture potential, production methods must be optimized and sustainable, cost-effective feeds and feeding strategies identified – in particular, research on fishmeal and fish oil sparing is a priority.
Fish oil is a valuable source of long-chain polyunsaturated fatty acids (LC-PUFAs) and is generally assumed to meet the essential fatty acid requirements of cultured fish for C18 PUFAs and/or LC-PUFAs. When fish oil is spared or completely replaced, essential fatty acid deficiencies may develop, particularly among fish that require LC-PUFAs. This has been reported for some species – cobia, white sea bass and California yellowtail – that grew reasonably well during studies with diets containing blends of fish oil and poultry fat but showed impairment on weight gain and feed conversion ratios among fish fed fish oil-free feeds.
LC-PUFAs are considered essential for many fish and individually have different biomechanical roles and/or physiological functions. Some fish can utilize C18 PUFA precursors as substrates to produce LC-PUFAs, while others – including many marine carnivores – cannot synthesize LC-PUFAs in significant amounts and depend on dietary sources for these bioactive fatty acids.
In this study, we evaluated the growth performance and tissue composition of juvenile California Yellowtail fed varying dietary levels of ARA, EPA and/or DHA. A quantitative estimate of dietary demand for critical omega-3 and omega-6 LC-PUFAs (i.e., ARA, EPA, and DHA) will help fish oil sparing and permit development of reduced or fish oil-free feeds that maintain physiological function and growth performance of cultured fish, especially those with LC-PUFA requirements. This article summarizes the original publication [Aquaculture 463 (2016) 123–134].

Study setup
The study was conducted at the Hubbs-Sea World Research Institute facilities (HSWRI) in Carlsbad, Calif., USA, using a semi-closed recirculating aquaculture system with 300-liter culture tanks, supplemental aeration, and mechanical (sand filters) and biological filtration.
The tanks were stocked with juvenile California Yellowtail (initial weight= 5.1 ± 0.1 gram, mean ±SE) and each dietary treatment was randomly assigned to three replicate tanks, siphoned daily to remove solids. Feeds were distributed continuously for 6 hours each day using commercial belt feeders and feeding rates used were slightly over apparent satiation estimates.

The experimental feeds were prepared at Center for Fisheries, Aquaculture and Aquatic Sciences (CFAAS; Carbondale, Ill., USA) per standard in-house procedures. As a basal formulation for all dietary treatments, we used a formulation based on previously validated practical feed formulations, which included 473.7 g/kg of commercial menhaden fishmeal. The primary lipid source in the positive control feed (FISH) was a commercial menhaden fish oil, and a commercial soybean oil was the primary lipid source in the negative control feed (SOY). We formulated the positive control to meet/exceed the known/assumed nutritional requirements of California Yellowtail, including the anticipated demand for LC-PUFAs supplied by fish oil and residual lipid associated with the fish meal used primarily as a protein source.
We also prepared additional experimental feeds by supplementing the soybean oil base with LC-PUFA concentrates containing high levels of EPA, DHA or ARA. Diets were formulated to contain suitable levels of the concentrates to reach dietary concentrations of ARA, EPA, DHA, ARA + DHA, or all three fatty acids at levels equivalent to 50 percent or 100 percent of the levels normally found in the FISH positive control feed. A total of 12 feeds were formulated as follows: FISH positive control, SOY negative control, SOY + 50 percent ARA, SOY + 100 percent ARA, SOY + 50 percent EPA, SOY + 100 percent EPA, SOY + 50 percent DHA, SOY + 100 percent DHA, SOY + 50 percent ARA and DHA, SOY + 100 percent ARA and DHA, SOY + 50 percent ALL, SOY + 100 percent ALL.
Diets were analyzed in triplicate to confirm proximate composition. Minor differences observed in dietary fatty acid composition were attributable to minute inaccuracies during the feed manufacturing procedure including ingredients weighing, incomplete mixing and/or sample collection.
At the end of the eight-week feeding trial, fish were counted and group-weighed by tank to assess performance in terms of weight gain, feed conversion ratio (FCR), specific growth rate (SGR), and feed intake. Samples of white muscle, liver, brain and eye tissues were collected from some fish for fatty acid analysis, and livers and total visceral masses were weighed to calculate hepatosomatic index (HSI) and viscerosomatic index (VSI).
For additional details on feeds preparation and analyses; experimental design and feeding trial; growth performance, sample collection and analysis; tissue fatty acid analysis; and statistical analysis, please refer to the original publication.
Results
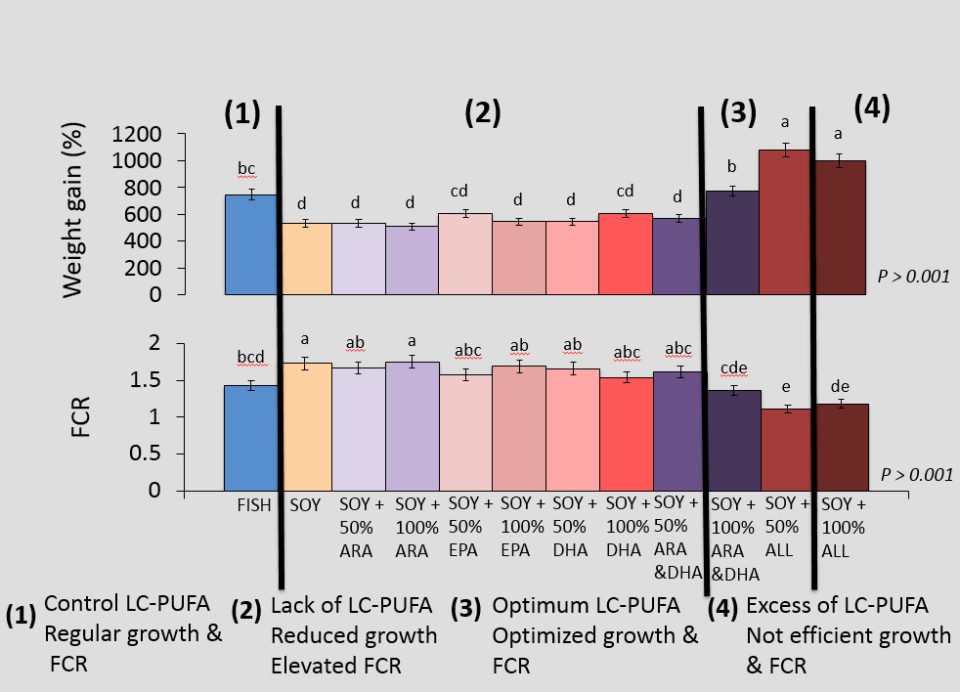
Results showed that the growth performance of juvenile California Yellowtail was influenced by dietary treatment (Table 1). Fish fed the SOY negative control feed had significantly reduced weight gain (532 ± 43 percent) and SGR (4.18 ± 0.14 percent body weight/day) and significantly higher FCR (1.73 ± 0.07) compared to fish fed the FISH positive control feed (weight gain = 747 ± 43 percent; SGR = 4.85 ± 0.14 percent body weight/ day; FCR = 1.43 ± 0.07). Also, complementing the soybean oil-based feeds with ARA alone had no effect on growth performance, irrespective of supplementation level (weight gain = 533 − 509 ± 43 percent, SGR = 4.18 − 4.10 ± 0.14 body weight/day, FCR = 1.67 − 1.75 ± 0.07).
Rombenso, California yellowtail, Table 1
| Production variables | FISH | SOY | SOY+50% ARA | SOY+100% ARA | SOY+50% EPA | SOY+100% EPA |
|---|
Production variables | FISH | SOY | SOY+50% ARA | SOY+100% ARA | SOY+50% EPA | SOY+100% EPA |
|---|---|---|---|---|---|---|
| Survival (%) | 49 ab | 52 ab | 55 ab | 44 b | 52 ab | 53 ab |
| Initial weight (g) | 5.1 | 5 | 5 | 5.1 | 5.1 | 5.1 |
| Final weight (g) | 43.0 bc | 31.9 d | 31.6 d | 31.3 d | 35.7 d | 33.1 d |
| Weight gain (%) | 747 bc | 532 d | 533 d | 509 d | 605 cd | 544 d |
| FCR | 1.43 bcd | 1.73 a | 1.67 ab | 1.75 a | 1.58 abc | 1.69 ab |
| SGR (% body weight/day) | 4.85 b | 4.18 c | 4.18 c | 4.10 c | 4.43 bc | 4.23 c |
| Feed intake (% body weight/day) | 8.93 | 8.82 | 8.53 | 8.73 | 8.68 | 8.82 |
| HSI | 1.3 | 1.8 | 1.7 | 1.6 | 1.4 | 1.8 |
| VSI | 8.1 | 9.4 | 9.3 | 9.7 | 8.1 | 8.3 |
FCR: feed conversion ratio; SGR: specific growth rate; HSI: hepatosomatic index; VSI: viscerosomatic index
Rombenso, California yellowtail, Table 2
| Production variables | SOY+50% DHA | SOY+100% DHA | SOY+50% ARA&DHA | SOY+100% ARA&DHA | SOY+50% ALL | SOY+100% ALL | PSE | P value |
|---|
Production variables | SOY+50% DHA | SOY+100% DHA | SOY+50% ARA&DHA | SOY+100% ARA&DHA | SOY+50% ALL | SOY+100% ALL | PSE | P value |
|---|---|---|---|---|---|---|---|---|
| Survival (%) | 52 ab | 55 ab | 52 ab | 59 a | 60 a | 60 a | 4 | 0.016 |
| Initial weight (g) | 5.1 | 5.1 | 5.1 | 5.1 | 5.1 | 5.3 | 0.1 | 0.425 |
| Final weight (g) | 32.9 d | 36.2 cd | 34.2 d | 44.4 b | 59.9 a | 58.2 a | 1.9 | <0.001 |
| Weight gain (%) | 544 d | 605 cd | 569 d | 774 b | 1080 a | 1001 a | 43 | <0.001 |
| FCR | 1.66 ab | 1.54 abc | 1.62 abc | 1.36 cde | 1.11 e | 1.18 de | 0.07 | <0.001 |
| SGR (% body weight/day) | 4.23 c | 4.43 bc | 4.31 c | 4.92 b | 5.621 a | 5.45 a | 0.14 | <0.001 |
| Feed intake (% body weight/day) | 8.71 | 8.53 | 8.63 | 8.72 | 8.5 | 8.6 | 0.16 | 0.246 |
| HSI | 1.5 | 1.4 | 1.7 | 1.3 | 1.4 | 1.2 | 0.3 | 0.367 |
| VSI | 9 | 9.2 | 9.1 | 9.7 | 9.3 | 8.9 | 0.6 | 0.165 |
FCR: feed conversion ratio; SGR: specific growth rate; HSI: hepatosomatic index; VSI: viscerosomatic index

However, supplementing individually with 50 percent EPA or 100 percent DHA restored production performance to that associated with the FISH positive control feed (weight gain = 605 − 605 ± 43 percent, SGR = 4.43 − 4.43 ± 0.14 percent bodyweight/day, FCR=1.58−1.54±0.07). Fish fed feeds jointly supplemented with ARA and DHA at the 100 percent level exhibited the same growth performance as those fed FISH feed. However, the soybean oil-based feeds supplemented with all three fatty acids improved growth performance of juvenile California Yellowtail beyond that associated with the FISH control, regardless of supplementation level (weight gain = 1080 − 1001 ± 43 percent, SGR = 5.61 − 5.45 ± 0.14 body weight/day, FCR = 1.11 − 1.18 ± 0.07).
Results of statistical analysis showed a stronger relationship between DHA with weight gain, and also between DHA with FCR than EPA or ARA; also, a more meaningful relationship between ARA and DHA with weight gain than ARA and EPA or EPA and DHA, and relatively weaker than all three LC-PUFAs.
Survival was significantly reduced in the SOY + 100 percent ARA group in comparison to SOY + 100 percent ARA and DHA, SOY + 50 percent ALL and SOY + 100 percent ALL groups; however, survival was relatively poor in all treatments (44 to 60 percent). HSI, VSI and feed intake did not vary among dietary treatments. Tissue fatty acid profiles were significantly affected by dietary treatments, with tissues largely reflecting the fatty acid profile of the diets. As expected, fillet and liver were more modified than eye and brain tissues.
Accumulation of 18:2 omega-6 at the expense of omega-3 LC-PUFAs was observed in all tissues of fish fed 18:2 omega-6-rich soybean oil-based feeds, reducing levels of EPA and DHA, particularly in the fillet and liver. Fillet and, in some cases, liver levels of ARA were also reduced among fish fed soybean oil-based feeds. However, soybean oil-based feeds supplemented with ARA, EPA, and/or DHA yielded tissues that were more similar to those of fish fed the FISH feed, at least with respect to these three LC-PUFAs.
To overcome the current difficulties related to fish oil sparing in feeds for marine carnivores, it is vital to understand and identify the essential fatty acid requirements of representative species. Our study showed that the use of soybean oil to completely replace fish oil in juvenile California yellowtail feeds is mainly controlled by the dietary availability of LC-PUFAs. Additionally, we present preliminary evidence that DHA and ARA are the principal drivers of LC-PUFA essentiality in California yellowtail. The species may be capable of synthesizing some LC-PUFAs de novo, but currently available information indicates that if any biosynthesis occurs in this species, it is insufficient to satisfy its physiological demand.
Perspectives
Results of our study show that soybean oil can totally replace fish oil in California yellowtail aquafeeds without affecting production performance and reducing LC-PUFA profile of fish tissues, if adequate levels of ARA and DHA are provided. Adding DHA, EPA and ARA may be of value regarding the improvement of production performance and maintaining tissue fatty acid profiles, but it looks like ARA and DHA are the primary drivers of LC-PUFA essentiality in this fish species.
References available from first author.
Authors
-

Artur Nishioka Rombenso, Ph.D.
Assistant Research Professor
Nutrition and Physiology Laboratory
Institute of Oceanography
Autonomous University of Baja California
Km 107 Carretera Tijuana-Ensenada
Baja California, Mexico 22860 -

Jesse T. Trushenski, Ph.D.
Director, Animal Health and Welfare
EVAQUA FARMS
4074 North 2000 East Road
Filer, Idaho 83328 USA -
David Jirsa, M.S.
Hubbs-SeaWorld Research Institute
2595 Ingraham Street
San Diego, California 92109 USA -
Mark Drawbridge, M.S.
Hubbs-SeaWorld Research Institute
2595 Ingraham Street
San Diego, California 92109 USA
Tagged With
Related Posts

Aquafeeds
A look at phospholipids in aquafeeds
Phospholipids are the major constituents of cell membranes and are vital to the normal function of every cell and organ. The inclusion of phospholipids in aquafeeds ensures increased growth, better survival and stress resistance, and prevention of skeletal deformities of larval and juvenile stages of fish and shellfish species.

Health & Welfare
Algae shows promise as alternative DHA source in rainbow trout diets
A growth trial in Canada evaluated the use of algae biomass to increase the concentration of long-chain polyunsaturated fatty acids in the tissues of rainbow trout.

Intelligence
Are omega-3s in farmed jade perch as high as believed?
Farmed jade perch – similar to other cultured fish species – is only rich in omega-3 fatty acids if its diet consists of these nutrients in high amounts. How does it compare to the wild fish?

Aquafeeds
Bridging the omega-3 gap with methane, microalgae
Innovation is leading to new ingredient options for renewable sources of omega-3 fatty acids. But Replicating long chain fatty acids is a tall order, Advocate contributor Lisa Duchene discovered.


