Technique to produce all-female, genetically protected populations
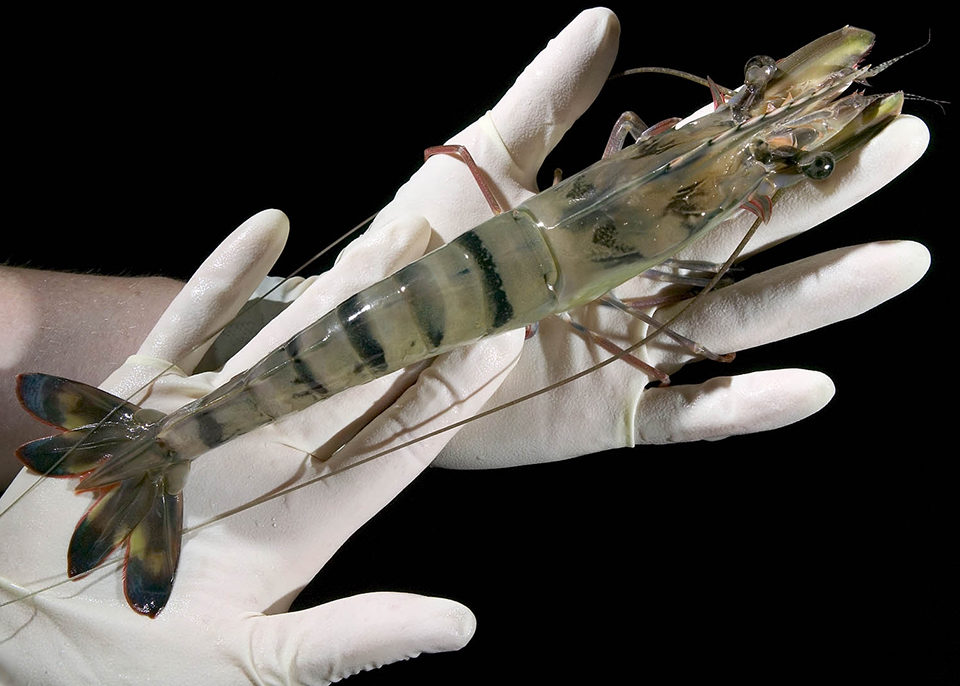
Australia has been at the forefront of developing elite stocks of giant black tiger shrimp (Penaeus monodon) with genetically improved lines under production in commercial farm ponds since 2006. The performance of these elite stocks has been consistently demonstrated, with growth and survival superior to that of wild-spawned progeny and incremental improvements over the past three years. The Australian industry is now ready to adopt new technologies to further improve farm productivity and provide a means to genetically protect breeders’rights and investments in genetic improvement.
Shrimp triploidy is currently the only known technology with the potential to provide the dual outcomes of all-female populations for improved productivity, as females are larger than males for all penaeid shrimp species, and reproductive sterility for genetic protection. With the potential to meet the future needs of the Australian shrimp industry, triploidy has therefore entered the initial phases of commercial trials.
Toward commercialization
Assessing the growth and survival performance of triploids in commercial rearing systems and comparing them to their known performance in experimental rearing systems is the first of several steps toward commercializing triploid technology. Knowledge of triploid shrimp performance in experimental controlled environment systems varies for different penaeid species.
The most comprehensively studied species are the Chinese shrimp (Fenneropenaeus chinensis) and Kuruma shrimp (Marsupenaeus japonicus). Following the recent advances in genetic improvement of black tiger shrimp, attention is now being directed toward that species.
Current challenges
Producing sufficient quantities of triploid black tiger shrimp seedstock for stocking commercial larval-rearing tanks and subsequent farm ponds by adapting
experimental induction techniques is the first key challenge to overcome to conduct commercial performance assessments.
Experimental induction techniques are typically performed on subsets of spawnings to allow comparisons to be made between different treatment and control protocols. As a result, embryo collection prior to induction through the application of a shock agent like hot seawater for experimental purposes is typically done by siphoning and collecting small quantities of embryos on a mesh screen.
However, to produce sufficient numbers of triploids for commercial-scale larval-rearing tanks, several whole spawnings must be induced to be triploid within a 24-hour time frame. To achieve this, we must develop a method to collect a whole spawning for triploid induction.
Shrimp embryos are extremely fragile immediately after being spawned and, if siphoned, handled or screened within the first six minuts after release, are easily damaged. Triploidy induction requires that the shock agent is applied to the embryos approximately eight minutes after release. This leaves only a two-minute window to collect an entire spawn, typically from volumes of seawater of 40 liters or more.
Siphoning at rates of 20 liters per minute or greater can damage shrimp embryos due to the water volume and pressure created, resulting in significant reductions in hatch rate and larval quality. Similarly, the addition of suitable volumes of heated seawater at a rate that results in the desired shock temperature also causes significant embryo damage and poor larval quality. Designing a system to apply the shock of hot water within a suitable time frame without causing embryonic damage is thus a key element of this challenge.
Once whole triploid spawning induction methods are developed, confirming the vigor of triploid larvae throughout the larval-rearing phase is the next research priority. Laboratory observations have showed the vigor of triploid larvae can be variable, with broodstock quality being a key influencing factor. High-quality broodstock are thus essential for inducing healthy triploid larvae.

Future challenges
Once satisfactory performance is demonstrated in the commercial larval-rearing and pond phases for triploid giant black tiger shrimp, the development of a fully automated induction process will be the next major step toward triploidy commercialization.
Australia’s Commonwealth Scientific and Industrial Research Organisation (CSIRO) has already developed an automated spawning detection device (ASDD) that will provide the first key component of an integrated triploid induction system. The ASDD reliably detects a spawning within one minute of eggs being released and often detects spawns while females are releasing the eggs. The ASDD provides an electrical output that can sound an audible alarm or send a message to a phone or pager once a spawning is detected.
If triploid shrimp are demonstrated to be viable in the commercial rearing systems, the ASDD can be used to stimulate a secondary system to automatically apply the hot seawater shock for triploid induction at a set time after spawning detection. The development of such a fully integrated automatic spawning detection and triploid induction system will require innovative engineering solutions catered to the biological fragility of the eggs.
The way forward
Industry-based research and development programs have been a key component in the success of Australia’s elite giant black tiger shrimp stocks. The authors believe that successful implementation and commercialization of triploid technology will likewise require close interactions among industry members, scientists and professionals of diverse disciplines.
The Australian Prawn Farmers Association and CSIRO’s Food Futures Flagship are now working together to assess the commercial potential of triploid giant tiger shrimp. Current efforts supported by the Australian Seafood Cooperative Research Centre are focused toward overcoming the initial challenge of inducing whole spawnings to produce healthy triploid seedstock.
(Editor’s Note: This article was originally published in the May/June 2009 print edition of the Global Aquaculture Advocate.)
Now that you've reached the end of the article ...
… please consider supporting GSA’s mission to advance responsible seafood practices through education, advocacy and third-party assurances. The Advocate aims to document the evolution of responsible seafood practices and share the expansive knowledge of our vast network of contributors.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year.
Not a GSA member? Join us.
Authors
-
Melony J. Sellars, Ph.D.
CSIRO Food Futures Flagship
CSIRO Marine and Atmospheric Research
233 Middle Street
Cleveland, Queensland 4163 Australia -
Andrew T. Wood
CSIRO Food Futures Flagship
CSIRO Marine and Atmospheric Research
233 Middle Street
Cleveland, Queensland 4163 Australia -
Brian Murphy
Gold Coast Marine Aquaculture
Woongoolba, Queensland, Australia
Tagged With
Related Posts

Health & Welfare
On the Job: Young fish veterinarian holds a hopeful outlook
Harry Hamlin-Wright is a fish vet. It’s a career choice that gets strange looks but offers him varied and interesting work. See the world of aquatic animal health and welfare through a fresh set of eyes in a new Advocate series on aquaculture jobs.

Health & Welfare
Triploid hard clams evaluated for Florida aquaculture
Induced triploidy could theoretically improve the survival of cultured hard clams because triploids divert energy from reproduction to somatic growth, resulting in larger and potentially hardier clams than diploids.

Health & Welfare
Reporting of fish escapes, causes crucial step in developing better controls
Escapes of fish from farm facilities can lead to interbreeding and competition for mating opportunities with wild fish. Escapees may also transmit diseases and parasites, as well as compete for feed and space.

Health & Welfare
A case for better shrimp nutrition
Shrimp farm performance can often be below realistic production standards. Use proven nutrition, feeds and feeding techniques to improve profitability.



