Precultured biofilms with sponge biocarrier treatments had lower levels of total ammonia nitrogen and nitrite, but higher nitrate concentrations

A main challenge in intensive aquaculture systems, including for Pacific white shrimp (Litopenaeus vannamei), is the accumulation of inorganic nitrogen – especially ammonia and nitrite – as a result of the animals’ feeding, which can have toxic effects and environmental impacts. Frequent water exchange to dilute toxic ammonia and nitrite concentrations is common as a management strategy, which can magnify the risk of infectious disease outbreaks. In addition, suspended solids generated by aquaculture processes can impact water treatment, environmental quality, animal welfare and public health and excessive suspended solids can affect the survival and growth of shrimp and must be managed.
In addition to recirculating aquaculture systems (RAS), the addition of submerged substrates in L. vannamei culture systems is another approach that maintains ideal water quality and enables low or zero water exchange rates and also significantly improves shrimp performance and decreases feed conversion ratios (FCRs).
Sponge biocarriers are widely considered ideal biocarriers for microbial attachment and growth due to their light weight, high hydrolysis stability and large specific surface area. In particular, sponge biocarriers have strong physical adsorption properties, which can remove suspended solids in aquaculture water bodies. In this study, sponge biocarriers with precultured biofilms (SBBFs) were used to set up and evaluate zero water exchange systems for L. vannamei.
This article – summarized from the original publication (Song, Z. 2023. Effect of Zero Water Exchange Systems for Litopenaeus vannamei Using Sponge Biocarriers to Control Inorganic Nitrogen and Suspended Solids Simultaneously. Sustainability 2023, 15(2), 1271) – presents the results of a study that evaluated the role of SBBFs in controlling inorganic nitrogen and suspended solids, and their impact on the growth performance of L. vannamei.
Study setup
The study was carried out at Qingdao Technical University (Qingdao, China). L. vannamei postlarvae (eight days old, PL8) shrimp were procured from a commercial hatchery, and after 18 days in the nursery, the shrimp had reached 1.6 ± 0.20 cm in length and 0.1 ± 0.001 grams in weight and were stocked into twelve 2-cubic-meter tanks filled with artificial seawater at 15 ppt and with mechanical aeration. The 2-cm sponge biocarriers utilized as the artificial substrate were packed in 60-by-60-cm net bags with a mesh size of 2 mm. A local, commercial nitrifying bacterial preparation was added to the system and various nutrients were also added to support the formation of biofilms.
Four treatments with three replicates each were tested: (1) sponge biocarrier control (SBC; 5 percent volume/volume, v/v); SBs without precultured biofilm and with aeration in a terylene (a form of polyester) net bag; (2) sponge biocarrier precultured biofilms (SBBF2.5a, 2.5 percent v/v); SBBFs and with aeration in a terylene net bag; (3) SBBF5a (5 percent v/v); SBBFs and with aeration in a terylene net bag; (4) SBBF5 (5 percent v/v), or SBBFs without aeration in a terylene net bag. The bags were submerged in the tanks throughout the experimental period.
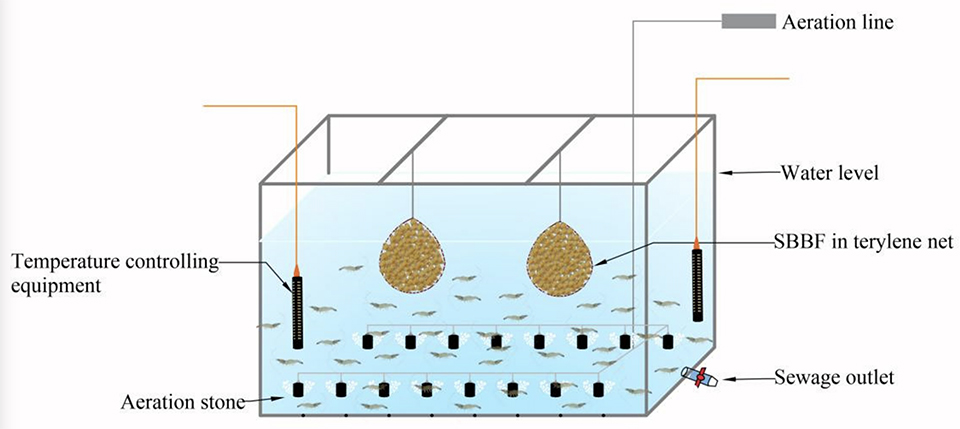
Shrimp were fed four times daily with local commercial feeds and shrimp growth and feed consumption were assessed daily and used to guide feed dosage. During the experiment, the water temperature was 28 ± 1.0 degrees-C, and DO was 6.5–8.0 mg per liter and any water volumes lost to evaporation and sampling were replaced. Every 10 to 15 days, the terylene net bags with SBs were removed from the tanks to remove the adsorbates in the pores and regenerate the sponge biocarriers’ adsorption capacity.
Results and discussion
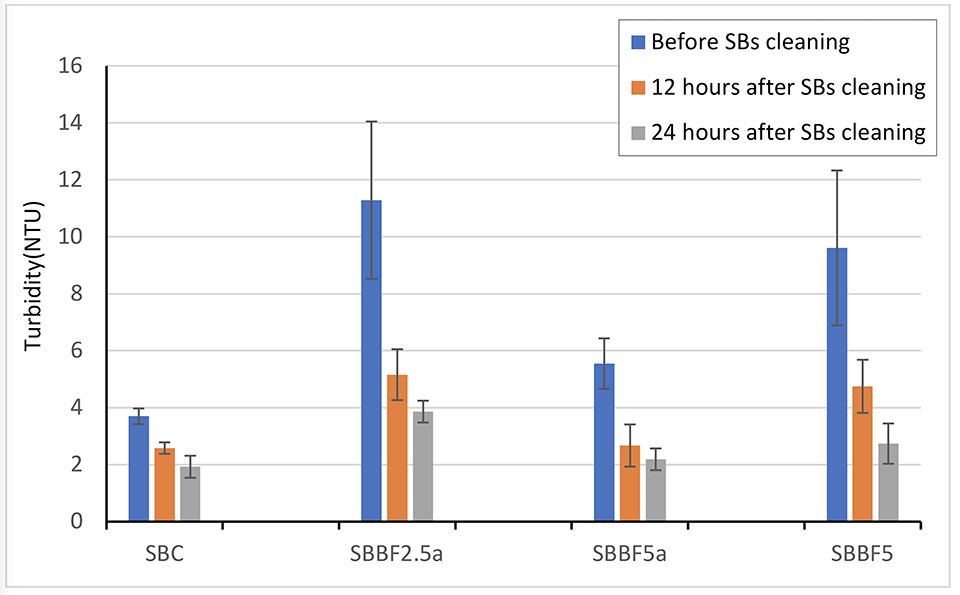
Water turbidity of all treatments was always maintained at a low level during the entire experiment, which was much lower than values reported for BFT systems. This result corroborates that the use of substrates can help particles attach to the biofilm, filter the water and reduce the suspended solids. The SBs added to the tanks have a strong adsorption capacity and can adsorb large amounts of SSs, which can be confirmed by the color change of the SBs, scanning electron microscopy imaging and dry weight increases. In another study, we found that the turbidity in shrimp culture tanks with SBs was significantly lower than that in water exchange treatments (WE), especially in the late stage of shrimp culture. The SBBF treatments had higher turbidity than the SBC treatments, which showed that the formation of biofilms can reduce the adsorption space of SBs.
The total ammonia nitrogen (TAN) concentrations of all four treatments were always lower than 0.3 mg per liter and remained below the safety levels for L. vannamei throughout the experiment. Although the SBC treatment had higher average and peak concentration of TAN than the SBBF treatments, they were much lower than those reported in shrimp cultures with artificial substrates and biofloc culture systems.
The difference in nitrite concentration between SBC and SBBF treatments was very significant. The SBC treatments also had significantly higher average and peak concentrations of nitrite than the SBBF treatments. Excessive nitrite accumulations are generally known to lower oxygen transport capability and weaken aquatic animal immune responses. This may be related to the short time of nitrite peak concentration, high dissolved oxygen concentration and high salinity.
Nitrate is the final product of the nitrification process. The level continuously accumulated during the shrimp culture period and the highest concentration was found in the SBBF5a treatments (98.99 mg per liter). However, in the SBC treatments, the highest concentration was only 13.43 mg per liter. The SBBF treatments exhibited a higher oxidation capacity of ammonium to nitrite and nitrate compared to the SBC treatments, and the oxidation performance increased with the percentage increase of the added SBBFs. SBBFs can almost immediately start nitrifying reactions once they have been deployed in tanks.

In this study, SBBF treatments involved precultured nitrifying biofilms, which oxidize ammonia to nitrate with nitrite as an intermediate via nitrification. Our experimental results confirmed that the nitrification process was effective with the addition of SBBFs and that the ammonia and nitrite removal processes occurred throughout the experimental period. This result can be confirmed by the changes in TAN, nitrite, nitrate concentration and pH reduction during the experiment. Although SBC treatments did not preculture biofilms, the concentrations of ammonia remained at safe levels for L. vannamei. This should be attributed to the small feeding amounts and shrimp excreta in the early stage of the experiment.
The use of artificial substrates has been reported to be associated with improved productivity in L. vannamei culture systems. The shrimp grown in our SBBF treatments exhibited a higher mean final weight, survival and productivity than those grown in the SBC. This benefited from the lower average and peak concentrations of TAN and nitrite nitrogen in the SBBF treatments. These values corroborate the findings of other studies that the presence of artificial substrates positively affects the performance of L. vannamei. But SBBF functions as a limited supplementary source of food are limited, and this may be related to the ability of SBs to adsorb some of the fine particles in the early stage of the experiment.
The unique feature of the zero-water-exchange systems used in this study is the integration of shrimp culture and water purification processes in the same tank, instead of circulating the production water through biofilters located outside the shrimp culture tanks, as is often done in typical RAS operations. The original aeration equipment in the aquaculture tanks was used to provide dissolved oxygen for nitrifying microorganisms on SBBFs.
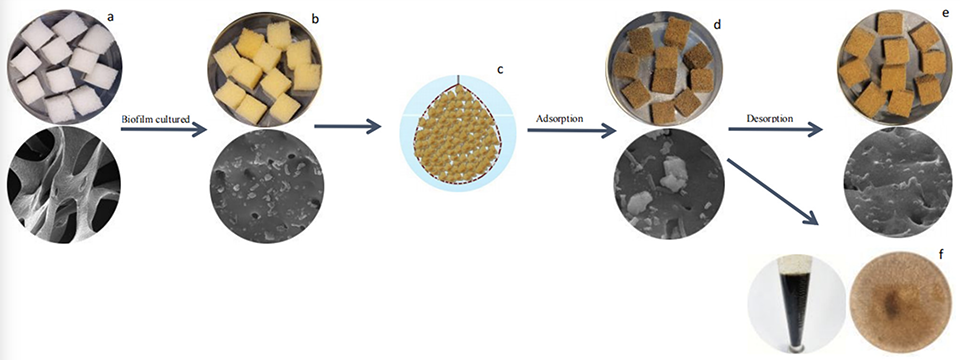
The systems can use tanks constructed of cement, canvas and other materials, or in containers; and without the biofilter, microfilter and protein separators used in conventional RAS. Each tank is an independent operational unit, which avoids the water circulation between different tanks used in many RAS operations. TAN and nitrite levels in the culture period are controlled by precultured nitrifying biofilms, so the lengthy start-up period related to the lower growth rate of nitrifying microorganisms is not necessary. Suspended solids are controlled by regular cleaning to remove the adsorbates of sponge biocarriers and anaerobic conditions inside the SBBF can be simultaneously prevented. In addition, this method does not require a start-up period and is inexpensive to build.
Further studies are needed to develop mechanical devices with automatic cleaning functions to improve the practicability of the SBBFs, and to evaluate the biofilm efficiency and the long-term stability of microbial community structure in large-scale culture systems.
Perspectives
We evaluated the role of SBBFs in controlling inorganic nitrogen and suspended solids and their impact on the growth performance of L. vannamei. The sponge biocarriers with precultured biofilms (SBBFs) in this study were used to build and develop zero water exchange systems for the shrimp.
The lower concentrations of ammonia and nitrite and higher concentration of nitrate revealed a more dynamic nitrifying process in the SBBF treatments than in the SBC treatments. The SBBF treatments can maintain the low levels of ammonia, nitrite, and suspended solids for L. vannamei under the strict requirement of zero water exchange during the entire culture period. The suspended solids produced during shrimp rearing in the aquaculture systems can be effectively controlled by progressive adsorption/desorption and by maintaining water turbidity within an acceptable range.
The L. vannamei grown in the SBBF treatments had higher mean final weight, survival and productivity than those grown in the SBC treatments. The feed conversion rate was lower in the SBC treatments than in the SBBF treatments.
Now that you've reached the end of the article ...
… please consider supporting GSA’s mission to advance responsible seafood practices through education, advocacy and third-party assurances. The Advocate aims to document the evolution of responsible seafood practices and share the expansive knowledge of our vast network of contributors.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year.
Not a GSA member? Join us.
Author
-
Dr. Zhiwen Song
Corresponding author
School of Environmental and Municipal Engineering, Qingdao Technological University, 777 Jialingjiang Rd., Qingdao 266000, China; and Key Laboratory of Eco-Environmental Engineer and Pollution Remediation in Shandong Province, 777 Jialingjiang Rd., Qingdao 266000, China
Tagged With
Related Posts

Health & Welfare
Ascidian, sponge culture supplies bioactive metabolites
In vitro culture is a viable method for supplying some ascidian and sponge metabolites for drug development and production.

Intelligence
Shrimp processing byproducts find many uses
Shrimp processing byproducts are constructively used in shrimp or fish feeds, livestock feeds and plant fertilizer. Chitin from shrimp exoskeletons is used in bandages and varied cosmetic products.
Innovation & Investment
Low-space bioreactors remove ammonia in recirculating systems
Despite their relatively small footprint, low-space bioreactors deliver sustainable and cost-effective biological wastewater treatment, particularly in recirculating aquaculture systems, where ammonia removal is critical.

Intelligence
Biofilters: Choosing your substrate
Heterotrophic and autotrophic bacterial communities supported within a biofilter naturally process organic wastes and provide biologically stable water.



