Ten-week study shows dietary supplementation of essential fatty acids may be required

In recent years, the aquaculture industry has faced a rapid shift in raw materials used in feed formulations with a departure from marine-based to (largely) terrestrial plant-based nutrient sources. Accurate information on ingredient nutrient (and anti-nutrient) composition and availability and the nutritional requirements of relevant species is a priority for the development of sustainable aquaculture.

It is broadly accepted that freshwater fish species are able to synthesize the omega-3, long-chain, highly unsaturated fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) from linolenic acid (LNA) to meet their physiological needs. However, positive responses to dietary supplementation of EPA- and DHA-rich oils have been reported in typical freshwater fry or juvenile fish, including channel catfish (Ictalurus punctatus) and hybrid tilapia (Oreochromis niloticus × O. aureus). The general paucity of scientific literature on fatty acid requirements and their roles in metabolism and immunity for a range of cultured fish species is a constraint for developing cost-effective feed formulations that optimize health and promote growth.
The largemouth bass (LMB), Micropterus salmoides, is one of several fish species of commercial importance in aquaculture for which conclusive evidence on the beneficial effects of dietary supplemental EPA- and DHA-rich ingredients remains very limited. Therefore, in view of the increased interest and expansion of LMB production for food fish markets in North America, our objective in this study was to assess the effects of supplemental EPA- and DHA-rich oils on the production performance, muscle fatty acid composition and the expression of genes related to metabolism and immunity of LMB. This research was supported by the U.S. Department of Agriculture Evans-Allen Research Program (Accession No. 1008396). The authors would like to thank all students of the Fish Nutrition Laboratory, Kentucky State University Division of Aquaculture for their assistance during this study.
Study setup
Twelve experimental diets were formulated to contain 45 percent crude protein (CP), 12 percent lipid, and different levels (0 percent, 1 percent and 2 percent) and ratios (20:80, 30:70, 40:60, 50:50, 60:40, 70:30, and 80:20) of EPA and DHA (Table 1). A diet depleted of omega-3 fatty acids (1; containing 0.27 percent n-3 fatty acids and 0.05 percent EPA+DHA) and a LNA-rich (4.35 percent) diet (2) were formulated using coconut and linseed oils, respectively.
Seven additional diets were formulated to contain 1 percent EPA+DHA at the aforementioned ratios using purified fish oils. A final set of three diets were formulated to contain 2 percent EPA+DHA at 20:80, 50:50, and 80:20 ratios. After diets were mixed, the experimental diets were screw-pressed through a 3-millimeter die plate using an industrial meat grinder, dried at room temperature under forced air, broken into pellets, sieved, and stored at minus-20 degrees-C until used.
Rossi, LMB trials, Table 1
| DIET | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|
DIET | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EPA+DHA (%) | 0.04 | 0.04 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 2.0 | 2.0 | 2.0 |
| EPA:DHA (%) | – | – | 20:80 | 30:70 | 40:60 | 50:50 | 60:40 | 70:30 | 80:20 | 20:80 | 50:50 | 80:20 |
| Protein | 45.2 | 45.6 | 45.2 | 46.4 | 46.1 | 45.7 | 45.6 | 45.7 | 45.7 | 45.6 | 46.0 | 44.9 |
| Lipid | 12.3 | 13.0 | 12.8 | 13.0 | 12.8 | 12.7 | 12.8 | 12.6 | 12.5 | 12.7 | 12.6 | 12.6 |
| Crude fiber | 1.8 | 3.4 | 2.0 | 2.1 | 2.2 | 2.4 | 2.0 | 3.5 | 2.4 | 2.5 | 2.8 | 2.5 |
| Ash | 13.3 | 13.7 | 13.7 | 13.6 | 13.5 | 13.3 | 13.6 | 13.7 | 14.0 | 13.7 | 13.8 | 13.5 |
| Fatty acids Sat | 3.95 | 2.46 | 2.40 | 2.37 | 2.35 | 2.30 | 2.30 | 2.26 | 2.24 | 2.29 | 2.05 | 2.15 |
| n-9 | 0.15 | 0.17 | 0.21 | 0.21 | 0.20 | 0.19 | 0.19 | 0.18 | 0.17 | 0.26 | 0.22 | 0.18 |
| n-6 | 2.46 | 3.52 | 3.55 | 3.58 | 3.59 | 3.52 | 3.56 | 3.55 | 3.52 | 3.58 | 4.02 | 3.71 |
| n-3 | 0.27 | 4.40 | 4.18 | 4.32 | 4.27 | 4.19 | 4.24 | 4.16 | 4.11 | 4.04 | 3.36 | 4.11 |
| n-6:n-3 | 9.02 | 0.80 | 0.85 | 0.83 | 0.84 | 0.84 | 0.84 | 0.84 | 0.86 | 0.89 | 1.20 | 0.90 |
| LNA | 0.21 | 4.35 | 3.27 | 3.34 | 3.30 | 3.24 | 3.26 | 3.20 | 3.14 | 2.18 | 1.79 | 2.20 |
| EPA | 0.02 | 0.02 | 0.21 | 0.31 | 0.40 | 0.49 | 0.59 | 0.66 | 0.75 | 0.43 | 0.79 | 1.50 |
| DHA | 0.03 | 0.02 | 0.67 | 0.63 | 0.54 | 0.44 | 0.36 | 0.27 | 0.20 | 1.36 | 0.73 | 0.38 |
| EPA+DHA | 0.05 | 0.04 | 0.88 | 0.94 | 0.94 | 0.92 | 0.95 | 0.94 | 0.95 | 1.80 | 1.52 | 1.87 |
| Final EPA:DHA ratio (%) | – | – | 23:77 | 33:67 | 43:57 | 53:47 | 62:38 | 71:29 | 79:21 | 24:76 | 52:48 | 80:20 |
LNA = linolenic acid; EPA = eicosapentaenoic acid; DHA = docosahexaenoic acid.
Feed-trained juvenile LMB (~5 g) were obtained (Cain Fish Farm, McCrory, Ark.) and transported to the Aquaculture Nutrition Laboratory located at the Aquaculture Research Center (Kentucky State University, Frankfort, Ky.). Following acclimation, fish were stocked into a 2,000-liter fiberglass tank operating as a recirculating aquaculture system (RAS) and fed with a commercial feed (45 percent CP, 15 percent lipid) until attaining adequate size for the feeding trial. Upon commencement of the feeding trial, groups of 15 LMB (average initial weight: 13.8 g) were sorted by hand and stocked into 36 110-liter glass aquaria operating as a RAS.

After a one-week conditioning period, experimental diets were randomly assigned to aquaria (in triplicate) and fish were fed twice daily to apparent satiation. Water quality parameters were maintained within acceptable ranges for LMB and a 12-hour photoperiod was employed using fluorescent lighting controlled by timers. At the end of the feeding trial, fish from each aquarium were group-weighed, counted, and a representative number of fish sampled for data collection. All resulting data was analyzed by orthogonal contrasts using statistical analysis system (SAS) software.
Results and discussion
The LMB juveniles readily accepted and fed actively on all experimental diets. We found no significant effects of EPA:DHA ratio on all response parameters evaluated (P<0.05). Results from a two-way analysis of variance on a subset of dietary treatments (3, 6, 9, and 10-12) representing a complete 2 × 3 factorial design reveled no effects of EPA and DHA level and ratio or their interaction (P<0.05; data not shown).
Likewise, we found no significant dietary effects after performing orthogonal contrast analyses of LMB final weight and feeding rates among selected treatments (Table 2). Despite excellent survival in all treatments, fish fed diet 2 containing high omega-3 and negligible EPA+DHA (0.05 percent) displayed lower survival than those fed diets 3-9 containing 0.9 percent EPA+DHA (P = 0.033).
Rossi, LMB trials, Table 2
| Diet | EPA+DHA (%) | Final weight (g) | Feeding rate (% BW/day) | Survival (%) |
|---|---|---|---|---|
| 1 | 0.05 | 107 ± 2.4 | 3.8 ± 0.09 | 97.8 ± 1.8 |
| 2 | 0.04 | 113 ± 4.1 | 3.9 ± 0.15 | 95.6 ± 3.6 |
| 3 to 9 | 0.93 ± 0.01 | 117 ± 0.9 | 3.7 ± 0.02 | 99.7 ± 0.2 |
| 10 to 12 | 1.73 ± 0.05 | 116 ± 1.0 | 3.8 ± 0.02 | 99.3 ± 0.4 |
| CONTRASTS (Pr > t) | ||||
| 2 – 1 | n/a | 0.391 | 0.470 | 0.369 |
| 2 – (3 to 9) | n/a | 0.417 | 0.187 | 0.033 |
| (3 to 9) – (10 to 12) | n/a | 0.788 | 0.355 | 0.720 |
n/a = not applicable. SE = standard error.
Interestingly, contrast analyses on weight gain (Fig. 1) and feed efficiency (Fig. 2) showed that LMB fed diets 3-9 outperformed those fed diet 2 (P<0.05), corroborating previous data that LMB have limited ability to synthesize EPA and DHA from LNA (Subhadra et al 2006). In addition, the lack of significant differences between dietary treatments 1 and 2 for all performance parameters evaluated (Table 2; Figs. 1 and 2) suggest that LMB has a low dietary LNA requirement.
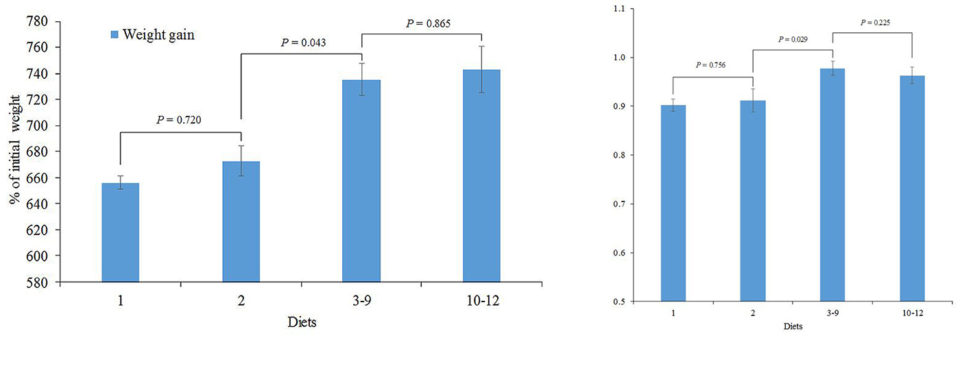
Fig. 2 (right): Feed efficiency of juvenile largemouth bass (13.8 g initial weight) after feeding the experimental diets for ten weeks. Connecting lines specify orthogonal contrasts between diets and/or diet groups. Error bars represent SE.
Perspectives

Although preliminary relative to all pending complementary results, our current findings indicate largemouth bass has a dietary requirement for EPA and/or DHA for maximal growth and nutrient utilization. The potential requirement will be investigated further in a dose-response feeding trial.
Further investigations to determine whether the observed responses were linked to age and growth rates of the fish, nutrient density (and performance) of the diets, or the combination thereof also are indicated.
References available from corresponding author.
Now that you've reached the end of the article ...
… please consider supporting GSA’s mission to advance responsible seafood practices through education, advocacy and third-party assurances. The Advocate aims to document the evolution of responsible seafood practices and share the expansive knowledge of our vast network of contributors.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year.
Not a GSA member? Join us.
Authors
-

Waldemar Rossi Jr., Ph.D.
Research Associate – Aquaculture Nutrition
Division of Aquaculture
Kentucky State University
103 Athletic Dr., Frankfort, KY 40601 USA -

Amit Y. Kumar
Graduate Research Assistant – Aquaculture Nutrition
Division of Aquaculture
Kentucky State University
103 Athletic Dr., Frankfort, KY 40601 USA -
Habte-Michael Habte-Tsion, Ph.D.
Researcher – Aquaculture Nutrition
Division of Aquaculture
Kentucky State University
103 Athletic Dr., Frankfort, KY 40601 USA
-
Kristy Allen, M.Sc.
Research Assistant – Aquaculture Nutrition
Division of Aquaculture
Kentucky State University
103 Athletic Dr., Frankfort, KY 40601 USA
-
Gagan D. Kolimadu
Graduate Research Assistant – Aquaculture Nutrition
Division of Aquaculture
Kentucky State University
103 Athletic Dr., Frankfort, KY 40601 USA -
James W. Tidwell, Ph.D.
Professor and Chair
Division of Aquaculture
Kentucky State University
103 Athletic Dr., Frankfort, KY 40601 USA -

Vikas Kumar, Ph.D.
Corresponding author
Assistant Professor – Aquaculture Nutrition
Division of Aquaculture, College of Agriculture, Food Science and Sustainable Systems (CAFSSS)
Kentucky State University
103 Athletic Dr., Frankfort, KY 40601 USA
Tagged With
Related Posts

Aquafeeds
Replacing fishmeal with DFB in pompano diets
A study evaluated the inclusion of bacterial, dried fermented biomass as a replacement for fishmeal in four practical diets for Florida pompano juveniles. There were no significant differences in final weight, survival, FCR or thermal-unit growth coefficient.

Health & Welfare
Dietary acidification in aquaculture
Much of the chemical breakdown of foodstuffs begins in the stomachs of animals through acidification. Effective use of digestive biology is a goal in aquaculture, so dietary acidifiers have been gaining interest in recent years.

Health & Welfare
Effect of dietary methionine on Pacific white shrimp juveniles
This study tested five diets formulated with increasing levels of methionine (Met) and Met + cysteine (Cys) and their effect on growth performance of juvenile Pacific white shrimp stocked at 50, 75 or 100 animals/m2 in a green water system.

Aquafeeds
The importance of carotenoids in aquafeeds
Carotenoids are important pigments that contribute characteristic quality criteria for the marketing of aquaculture products. Aquatic animals cannot biosynthesize carotenoids de novo, hence their inclusion in aquafeeds is important because they are associated with various metabolic functions.



