Study determines that geographic location of capture or aquaculture can influence the allergenic protein profiles of black tiger shrimp

Shellfish allergy has been an increasing health concern over the past decade. More than 2 percent of the general population is affected by food allergy to shellfish, with much higher prevalence in regions with high seafood consumption. Allergy to shellfish is often lifelong, similar to peanut allergy, and it can cause severe allergic reactions including anaphylaxis, therefore presenting a serious health risk to the individuals affected. More adults than children are affected by this food allergy, and shrimp seem to be the most implicated crustacean species. In the Asia-Pacific region, the prevalence of self-reported shellfish allergy ranges from 1 percent in children to 7.7 percent in adults.
Several allergenic proteins have been identified and characterized on a molecular level; however, the muscle protein tropomyosin is considered to be a major allergen found in most crustaceans and mollusks. Despite tropomyosin being the most common allergen, patient sensitization profiles to specific allergens have been found to differ geographically.
There is a lack of information on variability among allergens in animal-derived food sources. Recent proteomic studies on fish grown in aquaculture or caught in the wild have demonstrated variation in protein profiles. The quantitative differences reported by various researchers for major food allergens can directly impact the allergenicity of a food as well as food safety assessment.
In addition, currently, there is a lack of understanding of the range of variation in endogenous allergens in specific shellfish species, possibly impacting food safety assessment.
And while large variations in allergens have been demonstrated for different shellfish species, including shrimp and crab, the intraspecies variability of allergen expression in shrimp between different origins has not been investigated.
This article – summarized from the original publication (Dorney, R.D. et al. 2024. Variation in Shrimp Allergens: Place of Origin Effects on Food Safety Assessment. Int. J. Mol. Sci. 2024, 25(8), 4531 – discusses the results of a study that evaluated the impact of place of origin on the detection of the major allergen target tropomyosin, as well as eleven additional known crustacean allergens, in black tiger shrimp (Penaeus monodon), one of the most farmed and consumed shrimp species worldwide. Characterizing and comparing the allergen profiles of P. monodon from different geographical locations, as well as aquaculture and wild-caught specimens, may provide insight into the suitability of current crustacean food safety assessment tools.
Study setup
This study determined if the geographic location of capture, or aquaculture, influenced the allergenic protein profiles of black tiger shrimp. Sixty-three specimens of P. monodon, sized 10 to 14 cm, were collected from farmed and wild-caught shrimp from three Australian states (Western Australia, New South Wales and Queensland) and three Asian countries (China, India and Indonesia). All specimens were immediately frozen upon collection and transported to the research facilities involved in this study. Protein powder of individual specimens was prepared following published procedures.
The protein composition of samples was analyzed from the shrimp from the nine different locations in the Asia–Pacific by SDS-PAGE (an electrophoretic system commonly used as a method to separate proteins of certain molecular masses), immunoblotting (widely used analytical technique in molecular biology and immunogenetics to detect specific proteins in a sample of tissue homogenate or extract), and mass spectrometry (analytical technique that is used to measure the mass-to-charge ratio of ions).
For detailed information on the experimental design; sample collection, processing and analyses; and statistical analyses, refer to the original publication.
Results and discussion
Undeclared allergens present a serious health risk to allergic consumers because the management of shellfish allergy relies on strict food avoidance and correct food labeling. However, the basis for labeling in more than 180 countries is provided by the International Codex Alimentarius Commission, which explicitly specified in the “General Standard for the Labelling of Pre-packaged Foods” that crustacea and its subsequent products should always be declared. The vast majority of commercial detection systems for the quantification of crustacean allergens in food products target the major allergen tropomyosin.
Therefore, variation in the abundance as well as presence of different isoforms (a protein isoform is a member of a set of highly similar proteins that originate from a single gene or gene family and are the result of genetic differences) of tropomyosin may impact the detection and quantification of crustacean allergens in food products, therefore impacting food safety assessment. This study sought to characterize the quantitative differences in the major allergen target tropomyosin as well as eleven additional crustacean allergens of P. monodon sourced from different origins. In addition, isoform and variant diversity and the relative abundance of allergens were also investigated.
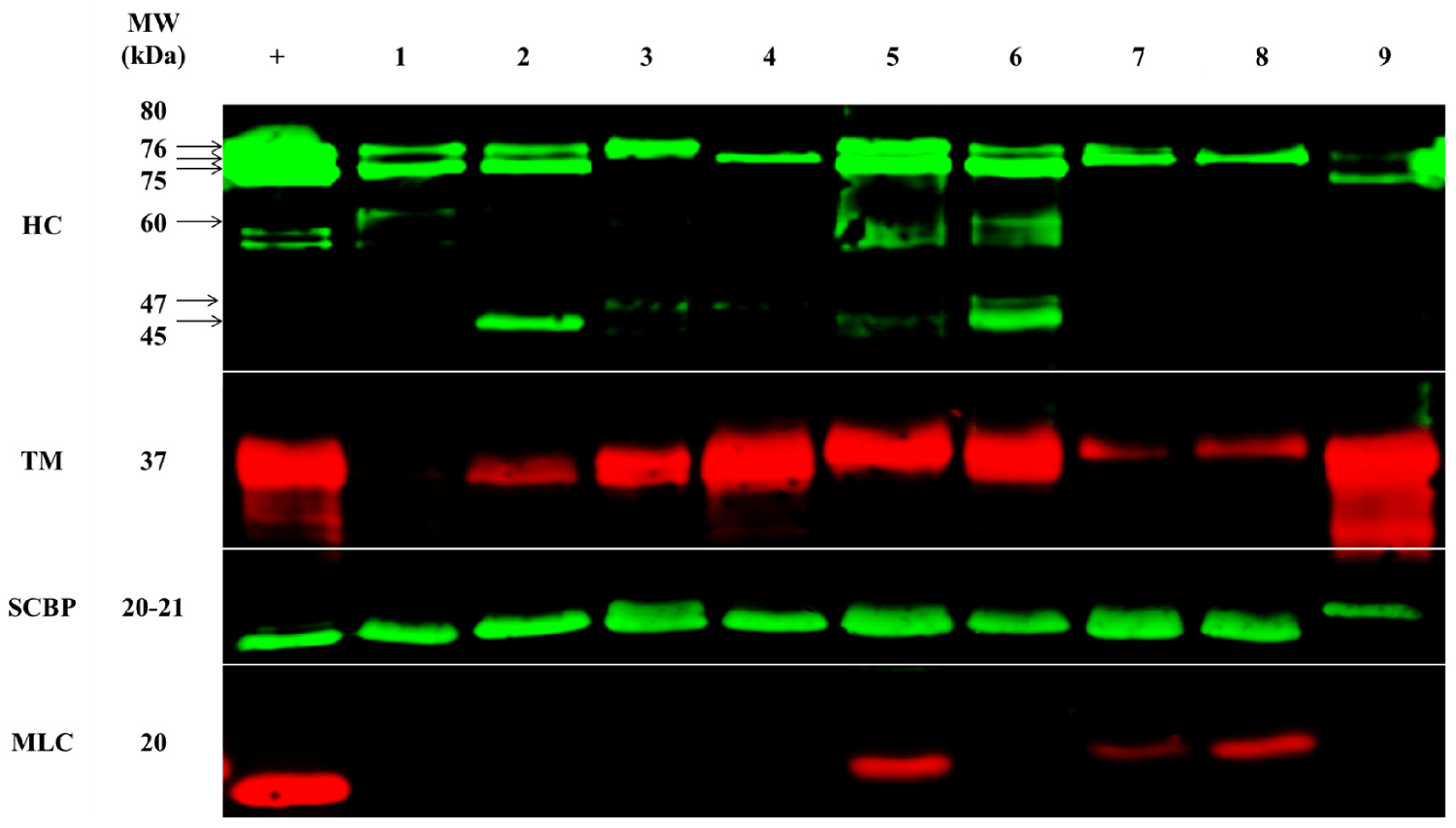
The clinical relevance of different allergens seems to be very dependent on the demographics of sensitized individuals. Sensitization to tropomyosin seems to be much lower in consumers from Asian countries, and researchers have suggested that this could be due to different eating habits and food processing. While Western populations mainly consume the muscle of shelled shrimp, East and Southeast Asian populations additionally consume other parts of shrimp, including the cephalothorax, which is rich in enzymatic proteins, as well as another allergen, hemocyanin (HC).
Transcriptomic-based approaches (techniques used to study an organism’s transcriptome, the sum of all of its RNA transcripts from their DNA) have shown that the expression of known crustacean allergens can vary between different shrimp species. Although no studies have investigated intraspecies variation in allergen profiles in relation to the provenance of shellfish, similar studies on fish have identified different protein profiles between farmed and wild fish.
To support the differences in allergen presence and abundance, we performed quantitative mass spectrometry. In total, ten of the twelve registered allergens were detected and their abundance was compared between origins: tropomyosin, arginine kinase (AK), myosin light chain 1 (MLC-1), myosin light chain 2 (MLC-2), sarcoplasmic calcium-binding protein (SCBP), troponin C (Tpn-C), troponin I (Tpn-I), triosephosphate isomerase (TpI), and glycogen phosphorylase (GP).
In general, heating (cooking) shrimp will usually result in a considerable degradation of labile allergens, and very heat-stable allergens such as tropomyosin seem to dominate. Most of the detected allergenic proteins in our study are stable to denaturation during heating. In our study, protein extracts were dried at a relatively low temperature of 60 degrees-C, and this seems to have resulted in the residual detection of various labile (constantly undergoing change). The stability of different shrimp allergens or even their less characterized isoforms in low temperatures has not been well studied, but it is expected to impact allergen detection and quantification.
While MLC was detected in very small amounts in only three locations, this protein had an abundant representation across all locations, as determined by using mass spectrometric (MS) analysis. In addition, the MS analysis was able to further distinguish between MLC-1 and MLC-2. MLC-1 was most predominant in China-farmed shrimp, but was similarly abundant across all locations, and MLC-2 was most predominant in China-wild shrimp, with some variation across locations. Although immunoblotting (widely used analytical technique in molecular biology and immunogenetics to detect specific proteins in a sample of tissue homogenate or extract) was unable to detect tropomyosin in Queensland-F shrimp, mass spectrometry did detect tropomyosin, although with very low abundance.
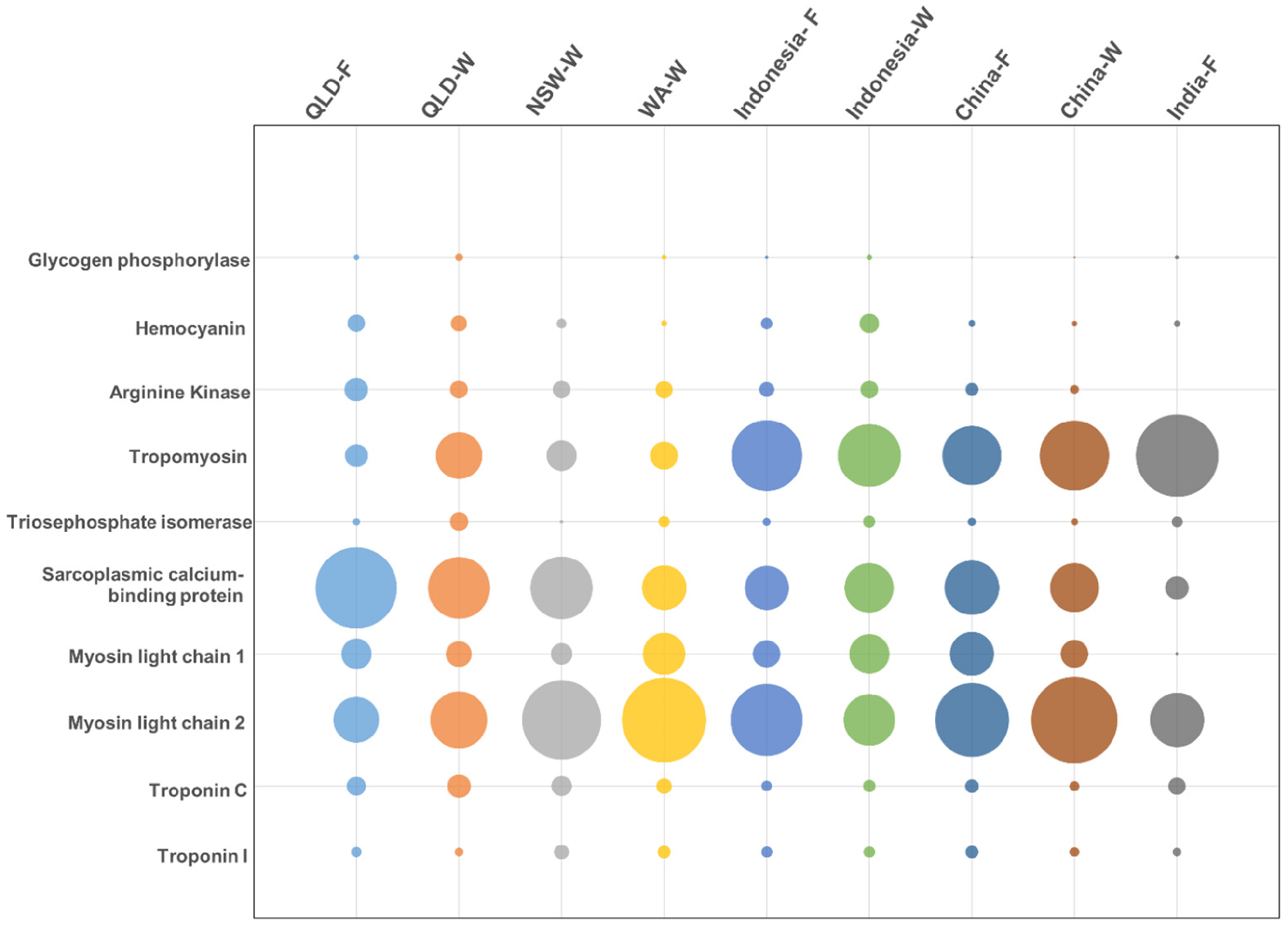
Similar to what was observed by immunoblotting, India-farmed shrimp had the highest relative abundance of tropomyosin, at nearly 13-times more compared to the lowest abundance in Queensland-farmed shrimp. Shrimp from Australia generally presented a lower tropomyosin abundance as compared to shrimp from other locations. There is little information to further elaborate on the mechanisms for the differences in the relative abundance of tropomyosin. Nevertheless, altered expressions of MLC-2 and tropomyosin have been associated with viral infection in two different shrimp species.
Data on the environmental conditions of the shrimp specimens were not collected, and thus the current study is limited in providing direct explanations for the proteomic differences observed between provenances. Future studies would benefit from data on factors such as temperature, salinity, and oxygen levels, as these have been shown to influence the expression of some prawn proteins, including recognized allergens such as hemocyanin and myosin light chain.
As the current study’s aim was to compare allergenic protein profiles between origins as well as assess proteomic differences and their potential influence on food safety, genomic analyses were not performed on the prawns. Therefore, the genetic contribution to observed proteomic differences is unknown; however, prawns are likely to exhibit genetic diversity between locations.
An analysis of single nucleotide polymorphisms (SNPs; a genomic variant at a single base position in the DNA) and population structure by various researchers suggests that geographically discrete P. monodon populations have likely undergone local adaptation to region-specific thermal regimes. Other studies on haplotype (group of genes inherited together by an organism from a single parent) diversity also support P. monodon population differentiation between different locations in the Indo-Pacific region, and also between aquaculture and wild-caught prawns. Although the current study does not have access to both wild-caught and aquaculture prawns from all locations, this is unlikely to greatly impact the findings of our study as aquaculture prawn populations are typically independent of their wild counterparts.
Perspectives
The findings of this study suggest that the allergen profile of P. monodon displays considerable intraspecies differences, as shown by variability in the levels of allergenic proteins as well as isoforms between populations from different origins. Tropomyosin demonstrated up to 13 times difference in abundance between origins, while MLC was consistently abundant in all shrimp. In general, tropomyosin appeared to be more abundant in farmed shrimp, while MLC-2 was most abundant in wild shrimp.
Because most commercial food allergen detection systems used on the food products for crustacean allergens target the protein tropomyosin, the results of this study show that the geographic origins of shrimp might directly impact the content level of this allergenic protein; thus, the readout of these commercial tests may be impacted and should be thoroughly considered in food safety assessment.
Future studies should aim to compare the allergenicity of P. monodon from various locations using specific antibodies to determine if these differences in the abundance of allergens impact allergenicity and food safety.
Now that you've reached the end of the article ...
… please consider supporting GSA’s mission to advance responsible seafood practices through education, advocacy and third-party assurances. The Advocate aims to document the evolution of responsible seafood practices and share the expansive knowledge of our vast network of contributors.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year.
Not a GSA member? Join us.
Author
-
Andreas L. Lopata, Ph.D.
Corresponding author
Molecular Allergy Research Laboratory, College of Public Health, Medical and Veterinary Sciences, Australian Institute of Tropical Health and Medicine, James Cook University, Townsville, QLD 4811, Australia
Tagged With
Related Posts

Aquafeeds
Nutritional disorders and aquatic animal health
Dietary nutritional disorders in farmed aquatic animals can be broadly defined as diet-related imbalances due to “under-” or “over-” nutrition.

Health & Welfare
Risks associated with chemicals and other agents used in attempts to control White Spot Syndrome Virus
Shrimp farmers in Asia and the Americas have used chemical and biological agents to control White Spot Syndrome Virus (WSSV) disease.

Intelligence
Warning: Shrimp salad may contain shrimp
Crustaceans, fish and any food that contains protein have the potential to cause allergic reactions in some individuals. To protect consumers, seafood businesses must stay abreast of changing regulations.

Health & Welfare
Seafood allergies: Control, prevention key to workplace management
Occupational seafood allergies can manifest as asthma, rhinitis, conjunctivitis, urticaria, and dermatitis. Anaphylactic reactions have also been reported.



