Results show potential to control emerging Vibrio spp. in shrimp aquaculture

Several Vibrio spp. have been associated with shrimp mortality and causing massive economic losses in countries that produce farmed shrimp. The most serious disease caused by Vibrio is Acute Hepatopancreatic Necrosis Disease (AHPND), and several Vibrio species have been determined to be involved, all of which commonly carry a 70 kbp plasmid (pVA1) that contains the PirA and PirB toxin genes.
The very limited efficiency of antibiotics and disinfectants to control these Vibrio spp. has led to a growing interest in the use of probiotics as epidemiological control tools. The use of probiotics is a common practice in current shrimp aquaculture. Namely, there are two main modes of action of probiotics – competitive exclusion and immunomodulation. Probiotics occupy and colonize the digestive tract, reducing the colonizer’s ability to colonize.
It is well known that probiotics can modify the intestinal microbiota in shrimp. Through the secretion of antibacterial substances, probiotics compete against pathogens, prevent their adhesion to the intestinal epithelium, compete for the necessary nutrients and produce antitoxic effects. There are numerous commercial probiotics, mainly based on bacterial strains of Lactobacillus and Bacillus.
Regardless of their efficacy and possible benefits for shrimp, many of these bacteria are not typical of marine environments, presenting problems for cultivation in shrimp farming systems for Pacific white shrimp (Penaeus vannamei), where they must compete against Vibrio spp. typical of the marine environment.
In the search for probiotic bacteria, typical of marine environments and capable of controlling Vibrio spp. of shrimp, it is essential to explore other bacterial genera typical of marine environments. Symbiotic bacteria from marine organisms could be ideal candidates, because they are adapted to marine conditions and can thrive in adverse environments. It is well known that sea sponges harbor very diverse microbial communities, but only a small group of these symbiotic bacteria can be cultured. The group of bacteria that is most amenable to culture are in the genus Pseudovibrio, and its species have been isolated from several marine invertebrates, flat worms, sponges and tunicates.
Pseudovibrio strains have the genomic potential to produce secondary metabolites and supply cofactors to the host. Among several molecules with different biological activities, the molecules with the most active antimicrobial activity are tropodithietic acid (TDA), alkaloids and polyketides like erythronolide.
Recently it was reported that low concentrations of TDA (0.5 μg/mL) exhibited activity against two pathogenic coral Vibrios. Because of the production of new compounds and the versatility of their metabolites, the biotechnological potential of the genus Pseudovibrio has been reported. Despite these properties, to date there are no reports on the use of Pseudovibrio spp. in shrimp aquaculture to control emerging pathogenic Vibrio spp.
This article – adapted and summarized from Aquaculture (Ecuador) Issue 130, August 2019 – reports on a study to determine the probiotic capacity in shrimp culture of several isolates of P. denitrificans isolated from the marine sponge Aplysina gerardogreeni collected in the El Pelado Marine Protected Area (REMAPE), Santa Elena, Ecuador.
Forty-three isolates of Pseudovibrio were obtained and in vitro competitive exclusion assays against Vibrio spp. were carried out. Most bioactive strains were validated by in vivo tests in the laboratory and in shrimp ponds. This article presents evidence of the probiotic qualities of several P. denitrificans isolates, highlighting their potential as a useful tool to control emerging Vibrio spp. in shrimp aquaculture.
Study setup
A total of nine samples of the A. gerardogreeni sea sponge were collected by diving in four rocky habitats at different depths from 10 to 30 meters in coastal waters of the Province from Santa Elena, Ecuador. Sponge samples were transported to the National Center for Marine Research (Centro Nacional de Investigaciones Marinas, CENAIM).
In the laboratory, macroscopic endobionts and epibionts were removed from sponge samples with scalpel and tweezers. Five grams of tissue taken from different parts of the body were macerated and diluted in 45-mL of sterile natural seawater (NSW). Subsequently, the homogenized samples were serially diluted to 10-5 in NSW. One hundred (100) μl of dilutions 10-2 to 10-5 were seeded in triplicate in Petri dishes containing Marine Agar 2216 (MA) BD DifcoTM. Plates were incubated at 27 degrees-C for two days. Subsequently, different morphotypes were selected and purified by successive stripes on Petri dishes containing MA. Pseudovibrio strains were initially selected based on their morphological and biochemical characteristics.
Subsequently, the taxonomy of isolates exhibiting bioactivity was determined based on nucleotide sequences of the 16 rRNA gene. With pure cultures of Pseudovibrio, aliquots were done in Luria-Bertani medium with NSW and 20 percent glycerol and stored at minus-80 degrees-C for subsequent tests.
For detailed information on the biochemical characterization of Pseudovibrio strains; in vitro evaluation of anti-Vibrio bioactivity; competitive exclusion test for punctual inoculation in agar; 16S rRNA gene sequencing amplification; safety assessment of P. denitrificans in the survival of P. vannamei postlarvae; effect of P. denitrificans on P. vannamei postlarvae exposed to Vibrio pathogens; effect of P. denitrificans on the survival of postlarvae and juvenile P. vannamei by challenge test with V. campbelli and V. parahaemolyticus; effect of competitive exclusion of P. denitrificans against luminescent V. harveyi in naturally infected P. vannameipostlarvae; effect of the application of Pseudovibrio in culture ponds of P. vannamei; bacterial biomass production; pool bioassay; and statistical analyzes, please refer to the original publication.
Results and discussion
Commercial probiotics used in shrimp farming belong predominantly to the genera Lactobacillus and Bacillus. Although some commercially used species are marine, most commercial probiotic bacteria are of terrestrial origin because they were initially formulated for other terrestrial animals. Although these bacterial formulations are proven effective for the original target organisms, their efficiency may be compromised when environmental conditions are very different, as in the case of marine shrimp crops. Penaeid shrimp are subject to high salinity and temperature ranges, which favors the growth of Vibrio spp.
The optimal range of salinity and temperature to culture P. vannamei is 20 to 35 ups and between 22 and 32 degrees-C. Using metagenomics, Zhang et al. (2016), showed that salinity modifies the microbiota in shrimp, finding that the higher the salinity, the more dominant the Vibrio are to the detriment of Lactobacillus, while Vezzulli et al. (2013), report that a temperature below 37 degrees-C negatively affects the growth of Lactobacillus.
Marine biodiscovery (the collection of samples of native biological materials – e.g. plants, animals and other organisms – to test for compounds that may have commercial applications, like new pharmaceuticals and insecticides) is a promising alternative to isolate marine bacteria that function as effective probiotics for marine organisms that can be cultured. Marine invertebrates, particularly sponges, harbor great bacterial diversity. Among the marine bacteria that can be cultured, the genus Pseudovibrio stands out, and in recent decades has been the subject of studies due to its versatility to produce bioactive molecules against Gram-negative and Gram-positive bacteria.
In this study, we separated several isolates of P. denitrificans which exhibit probiotic qualities for shrimp, due to their strong anti-Vibrio bioactivity. These qualities were demonstrated in our experiments, challenge tests in laboratories and in culture ponds.
Dominguez, Vibrios, Table 1
| Treatments | Stocking density (per square meter) | Average weight (g) | Survival (%) | Yield (kg/ha) | FCR |
|---|
Treatments | Stocking density (per square meter) | Average weight (g) | Survival (%) | Yield (kg/ha) | FCR |
|---|---|---|---|---|---|
| Ps-17 | 8 | 10.2±2.0 a | 79.2±5.3 a | 899.6±59.5 a | 0.99±0.16 a |
| Ps-18 | 8 | 10.8±3.4 a | 66.3±6.8 b | 753.4±77.2 b | 1.14±0.28 a |
| Control | 8 | 9.4±2.4 a | 63.0±6.7 b | 715.9±76.4 b | 1.35±0.32 a |
We found bioactivity against V. parahaemolyticus, V. campbellii, V. vulnificus and V. harveyi and against the highly virulent pathogenic strain of V. parahaemolyticus BA94C2 positive for PirA / PirB, toxins associated with AHPND pathologies in shrimp culture. As far as we know, this is the first report that analyzes the anti-Vibrio potential of Pseudovibrio against Vibrio spp. in shrimp. The antibacterial activity of the genus Pseudovibrio has been mentioned in numerous studies, against Gram-positives and Gram-negative bacteria, such as E. coli, Bacillus subtilis, Kluyveromyces marxianus, Salmonella enterica serotype Typhimurium, Staphylococcus aureus resistant to methicillin (MRSA) and Clostridium difficile.
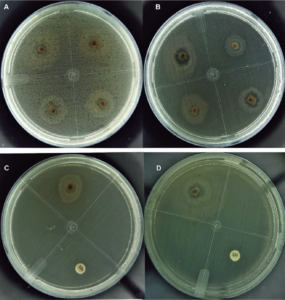
Recently, activity was reported against two Vibrio pathogens found in corals, Vibrio coralliilyticus and Vibrio owensii. The antibacterial activity of Pseudovibrio strains has been associated with their metabolome, sulfuric acid, and tropodithietic acid (TDA). TDA can kill or inhibit Vibrio spp. pathogens in fish larvae. In addition, salinity favors the growth of Pseudovibrio because it is a bacterium typical of marine environments, and our Pseudovibrio isolates had an optimal growth between 25 to 31 degrees-C. These conditions favor their ability to compete against Vibrio spp.
A very important step in assessing probiotic characteristics is to rule out the safety of bacterial candidates. Our results corroborate the safety of three Pseudovibrio isolates evaluated in zoea-1 shrimp larvae. The microbiological analysis confirmed that the Pseudovibrio were associated with zoea-1 without causing adverse effects. Several Pseudovibrio spp. have been reported as symbionts (organisms living in symbiosis, any type of a close and long-term biological interaction between two different biological organisms) of many marine benthic invertebrates, including shrimp. In addition, Pseudovibrio is well equipped to survive in adverse environments where nutrients can fluctuate, such as shallow seawater, and can proliferate in ultra-oligotrophic (low nutrient levels) marine waters.
This metabolic versatility of the genus favors its association with its invertebrate hosts and is beneficial or at least neutral, because most of them are commonly associated with healthy animals. After the safety tests, the next step was to determine the effectiveness of P. denitrificans against Vibrio spp. using in vivo tests.
To verify the effectiveness of P. denitrificans as a probiotic, two challenge tests were performed using two highly virulent vibrios, V. campbellii and V. parahaemolyticus, resulting in a significant increase in the survival of challenged larvae and juveniles. In addition, the tests were conducted at the PL3 (3-day postlarvae) stage, which was already naturally infected with V. harveyi, strongly indicating the ability of P. denitrificans to compete and displace Vibrio spp. present in shrimp postlarva cultures and improving PL survival.

This finding is very relevant, considering that bacteria of the genus Vibrio are quite adapted to the culture environment of marine organisms. There were no significant differences in the survival of shrimp larvae treated with Ps11, Ps17 and Ps18 when compared to the control group. The high mortality observed in the shrimp postlarvae in the control group was probably related to the high load of luminescent V. harveyi present.
Luminescent species of Vibrio have been associated with severe mortalities in shrimp farming, mainly in the nurseries in shrimp farms. The pond test confirmed the beneficial effects of the application of P. denitrificans, demonstrating its effectiveness to increase production, survival, average shrimp harvest weight and yields. The best production yield was recorded with P. denitrificans isolate Ps17, compared to isolated Ps18. This result indicates that the P. denitrificans isolates tested may have different effects and, therefore, caution should be exercised when extrapolating the beneficial effect of P. denitrificans strains.
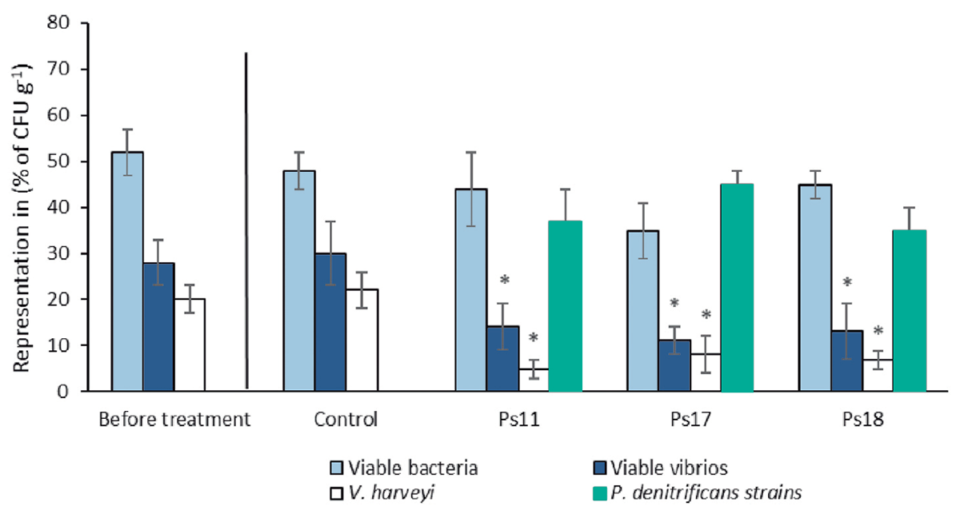
The variability in the resulting bioactivity obtained from different isolates indicates the need to carry out similar studies using several isolates. In our study, we used nine sponge samples collected in different places to increase the possibility of obtaining a large genetic set of bacteria. Similar results have been found in other bacteria. Particularly, the strain (ILI) of V. algynoliticus functions as a probiotic for shrimp, while other strains of the same species have been reported as pathogenic. Similarly, Sonnenschein et al. (2018), reports toxicity of different strains of Roseobacter against microalgae.
Perspectives
Marine biodiscovery is a promising alternative to isolate marine bacteria that can be used as effective probiotics for cultured marine organisms. To identify Pseudovibrio spp. that could be highly bioactive against the pathogenic Vibrio spp. of shrimp, several samples of different morphotypes of the sponge A. gerardogreeni were collected. From these, 43 isolates were obtained including three isolates – coded as Ps11, Ps17 and Ps18 – that showed the highest bioactivity against Vibrio spp. that are highly pathogenic to shrimp.
The anti-Vibrio bioactivity demonstrated by in vitro tests was confirmed by the beneficial effects observed in the challenge tests, the naturally infected larvae and in the shrimp culture ponds. The results of our study indicate which Pseudovibrio can be used as a biological control for Vibrio spp. in shrimp culture. More studies are needed to evaluate practical concentrations on a commercial scale.
References available from the original publication.
Now that you've reached the end of the article ...
… please consider supporting GSA’s mission to advance responsible seafood practices through education, advocacy and third-party assurances. The Advocate aims to document the evolution of responsible seafood practices and share the expansive knowledge of our vast network of contributors.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year.
Not a GSA member? Join us.
Authors
-
Cristóbal Domínguez-Borbor
ESPOL Polytechnic University
Escuela Superior Politécnica del Litoral, ESPOL
Centro Nacional de Investigaciones Marinas (CENAIM)
Campus Gustavo Galindo Km. 30.5 Vía Perimetral
P.O. Box 09-01-5863. Guayaquil, Ecuador -
Valeska Ardiles
ESPOL Polytechnic University
Escuela Superior Politécnica del Litoral, ESPOL
Centro Nacional de Investigaciones Marinas (CENAIM)
Campus Gustavo Galindo Km. 30.5 Vía Perimetral
P.O. Box 09-01-5863. Guayaquil, Ecuador; and
Pontificia Universidad Católica de Valparaíso
Avenida Altamirano 1480, Valparaíso, Chile -
Marissa Bermeo
ESPOL Polytechnic University
Escuela Superior Politécnica del Litoral, ESPOL
Centro Nacional de Investigaciones Marinas (CENAIM)
Campus Gustavo Galindo Km. 30.5 Vía Perimetral
P.O. Box 09-01-5863. Guayaquil, Ecuador -
Chalén Bolívar-Alvarado
ESPOL Polytechnic University
Escuela Superior Politécnica del Litoral, ESPOL
Centro Nacional de Investigaciones Marinas (CENAIM)
Campus Gustavo Galindo Km. 30.5 Vía Perimetral
P.O. Box 09-01-5863. Guayaquil, Ecuador -
Cecilia Tomalá
ESPOL Polytechnic University
Escuela Superior Politécnica del Litoral, ESPOL
Centro Nacional de Investigaciones Marinas (CENAIM)
Campus Gustavo Galindo Km. 30.5 Vía Perimetral
P.O. Box 09-01-5863. Guayaquil, Ecuador -
Stanislaus Sonnenholzner, Ph.D.
ESPOL Polytechnic University
Escuela Superior Politécnica del Litoral, ESPOL
Centro Nacional de Investigaciones Marinas (CENAIM)
Campus Gustavo Galindo Km. 30.5 Vía Perimetral
P.O. Box 09-01-5863. Guayaquil, Ecuador -
Jenny A. Rodríguez, Ph.D.
Corresponding author
ESPOL Polytechnic University
Escuela Superior Politécnica del Litoral, ESPOL
Centro Nacional de Investigaciones Marinas (CENAIM)
Campus Gustavo Galindo Km. 30.5 Vía Perimetral
P.O. Box 09-01-5863. Guayaquil, Ecuador
Tagged With
Related Posts

Health & Welfare
AHPN inferences based on behavior of vibrio bacteria
Vibrio parahaemolyticus, a strain of which is the cause of acute hepatopancreatic necrosis (AHPN), has both virulent and benign strains. This strain colonizes the stomachs of shrimp by the formation of a biofilm, which protects it from antibiotics and other potential treatments.

Intelligence
As ocean temperatures rise, so too will vibrio outbreaks
A study using a half-century of data has linked climate change and warming sea temperatures with an increase in illnesses from the common vibrio bacteria. Shellfish growers, fighting a particularly virulent strain of Vibrio parahaemolyticus, are changing their harvest protocols.

Health & Welfare
A holistic management approach to EMS
Early Mortality Syndrome has devastated farmed shrimp in Asia and Latin America. With better understanding of the pathogen and the development and improvement of novel strategies, shrimp farmers are now able to better manage the disease.

Health & Welfare
Clinical case report: EMS/AHPND outbreak in Latin America
Behind the successful control of Early Mortality Syndrome (EMS), or Acute Hepatopancreatic Necrosis Disease (AHPND), in a Latin American shrimp nursery.


