Dietary inclusion of Aurantiochytrium sp. meal increased the resistance of P. vannamei challenged with White Spot Syndrome Virus
Temperature is one of the main environmental factors in Pacific white shrimp (Penaeus vannamei) farming. Low or suboptimal temperatures due to cold fronts during certain seasons of the year are challenging for shrimp production, as they are ectothermic (cold-blooded) organisms that thermoregulate inefficiently and at a high energy cost. The ideal temperature range for the species is between 27–30 degrees-C and values outside this optimal range can increase susceptibility to diseases, including White Spot Syndrome Virus (WSSV).
Crustaceans are incapable of synthesizing long-chain polyunsaturated fatty acids (LC-PUFAs) so there is a dietary requirement for these very important fatty acids. This inclusion may have beneficial physiological effects, particularly under low temperatures, and perform important functions directly linked to the immune system and inflammatory responses. PUFAs are produced by marine microorganisms, macroalgae, and microalgae.
Protists (eukaryotic organisms, those whose cells have a membrane-bound nucleus and that are not an animal, plant, or fungus) like Aurantiochytrium sp. have a high production capability for LC-PUFAs, especially docosahexaenoic acid (DHA). Various species are major producers of fatty acids and have been widely used in diets for aquatic organisms in recent years. Thus, the inclusion of Aurantiochytrium sp. as a feed additive in practical diets for Pacific white shrimp could be relevant for the animals in periods of cold stress, helping to deal with suboptimal temperatures and protecting against diseases.
This article – summarized from the original publication (Hoffling, F.B. et al. 2024. Aurantiochytrium sp. Meal as Feed Additive for Pacific White Shrimp Reared under Low Temperature and Challenged by WSSV in Association with Thermal Stress. Fishes 2024, 9(3), 108) – presents the results of a study that evaluated the performance of P. vannamei juveniles receiving five dietary treatments with 0, 1, 2, 3, and 4 percent inclusions of Aurantiochytrium sp. meal in a clean water system at a suboptimal temperature (22 degrees-C) regarding zootechnical, immunological, and microbiological parameters, and a WSSV challenge.
Study setup
This rearing study was conducted at the Laboratório de Camarões Marinhos, Federal University of Santa Catarina in Florianópolis, Brazil. It tested the inclusion of Aurantiochytrium sp. meal in the rearing of P. vannamei grown in a clear water system and at a suboptimal temperature of 22 degrees-C. The WSSV challenge was carried out at the Instituto Federal Catarinense in Araquari, Brazil. The shrimp postlarvae (PL10) were procured from the company Aquatec (Rio Grande do Norte state, Brazil) and reared in a greenhouse biofloc system until they reached an average weight of 3.8 ± 0.02 grams.
The nine-week growth trial was then conducted in an indoor clean water system with 80–100 percent daily water change. Six hundred shrimp were transferred to the clean water system at 22 degrees-C and stocked at 100 shrimp per cubic meter in 400-liter tanks.
The experiment involved testing five dietary treatments formulated according to the nutritional requirements of P. vannamei to be isonitrogenous and isoenergetic. The control diet contained soy lecithin and fish oil to meet the nutritional requirements of P. vannamei for phospholipids. with 0, 1, 2, 3, and 4 percent of Aurantiochytrium sp. meal, and treatments were run in triplicate. Room temperature was controlled using air conditioning and water was cooled using a central chiller (22 degrees-C) to replace static water.
For detailed information on the experimental design, animal husbandry, data collection and analyses, refer to the original publication.
Is the key to predicting harmful algal blooms found in genetics?
Results and discussion
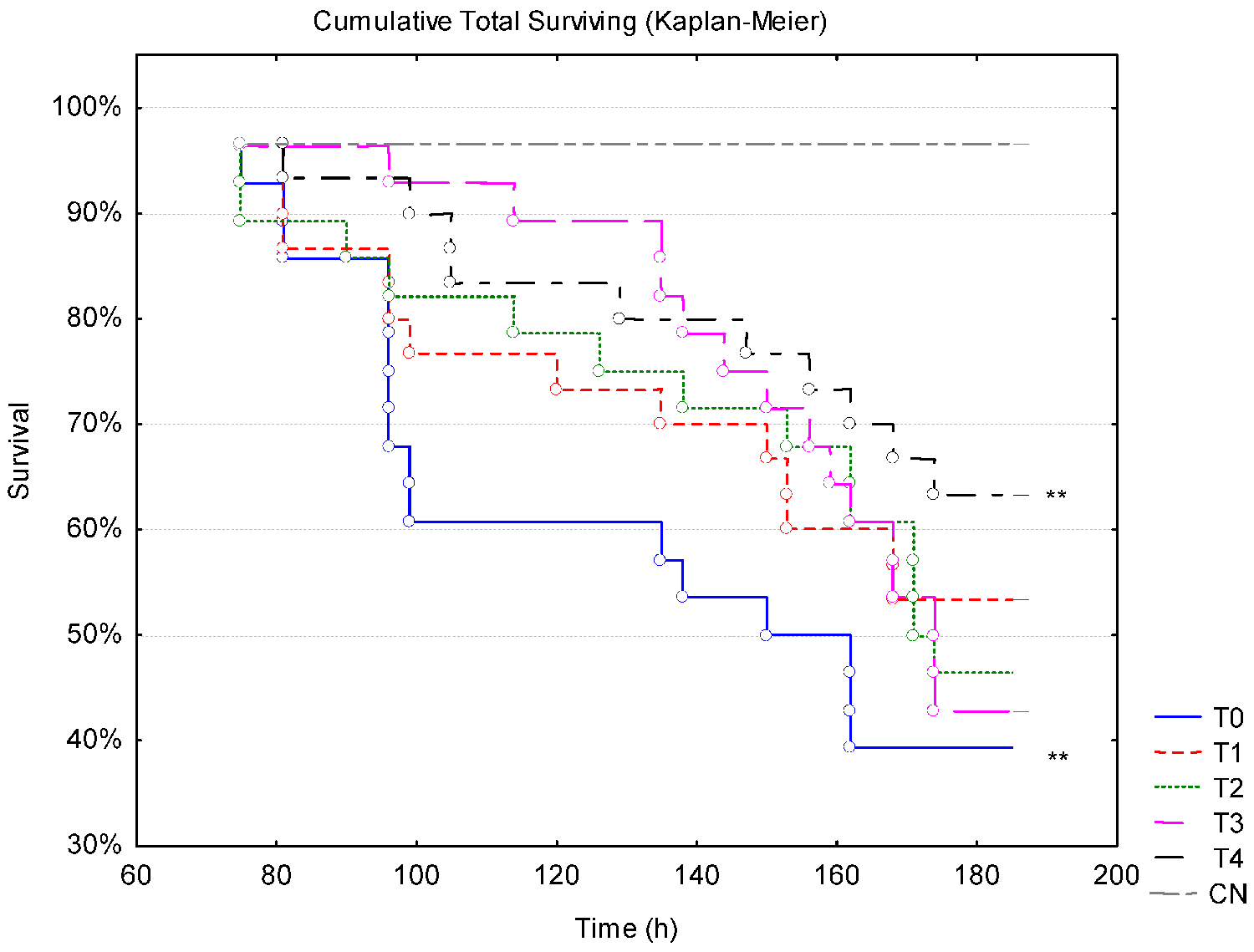
During the entire rearing period, the animals from the different dietary treatments were kept under a suboptimal water temperature of 22 degrees-C. However, their final survival was not affected by this temperature, remaining above 96.7 percent in shrimp from all dietary treatments. This high survival demonstrates the adaptability of P. vannamei to the temperature range of 22 degrees-C and the importance of not having rapid fluctuations in temperature, despite being at a temperature below the ideal recommended for the species. No statistical differences were identified regarding the growth performance parameters among the treatments.
Shrimp can activate some metabolic regulatory pathways when facing temperature fluctuations, and the presence of dietary phospholipids may have contributed to the shrimp response to cold, as reported by other researchers. In the present study, shrimp fed diets with the inclusion of 1, 2, 3, and 4 percent Aurantiochytrium sp. meal had DHA contents of 0.48, 0.73, 0.91, and 1.11 percent, respectively, while shrimp fed the control diet had about 0.25 percent DHA, corroborating the results of other studies where the inclusion of soy lecithin in all diets may have reduced the DHA requirement of shrimp.
The suboptimal temperature in our study, the increase of the dietary n-3:n-6 ratio in fatty acids, and the addition of the ingredient rich in DHA and PUFAs beyond the known requirement had no adverse effects on shrimp survival and growth. On the contrary, the shrimp appeared to adapt well to the low temperature in a system without temperature fluctuations, and no differences in growth performance were observed even when the n-3:n-6 ratio was increased with the increase in feed additive dose.
The feeding behavior of farmed shrimp is typically affected by low water temperatures, with reduced feed intake and animals remaining immobile. However, this was not the case in our study, as the shrimp exhibited relatively active feeding behavior and feed consumption. This may be related to the stable temperature during the experiment and the adequate temperature acclimatization of the animals prior to the experiment. But the suboptimal temperature did impact the feeding rate, which was determined to be less efficient when compared to other studies carried out at ideal, higher temperatures.
There were no significant differences in the quantification of total heterotrophic and total Vibrio bacteria, which naturally occur in the marine environment and intestinal tract of shrimp. The water exchanges were performed daily at rates of 80–100 percent of the total volume, a factor that influenced the maintenance of low levels of nitrogenous compounds in the system, possibly helping in controlling the proliferation of Vibrio. These bacteria can grow over a wide range of temperatures (0.5 to 48 degrees-C), but there is evidence that their growth rate is accelerated at higher temperatures, such as for V. parahaemolyticus and V. alginolyticus at 37 to 42 degrees-C. The total bacteria count we observed is in line with results found in previous studies.
Regarding the challenge trial, at the low temperature of 22 degrees-C we observed that supplementation with Aurantiochytrium sp. meal at concentrations of 3 and 4 percent increased the resistance of the shrimp to viral infections, being statistically significant when compared to the control group (0 percent). However, when the temperature increased to 28 degrees-C during the challenge, the group supplemented with 4 percent Aurantiochytrium sp. meal was the only one that had greater survival and therefore greater resistance to WSSV.
This finding may be associated with the composition of Aurantiochytrium sp. and the beta-1,3-glycans in its cell wall. This polysaccharide has properties that promote animal health, such as antioxidant, anti-inflammatory, and immunostimulant activities. In marine shrimp, various studies have shown that dietary supplementation of beta-1,3-glycans promoted greater resistance against WSSV infection, and our results are similar.
Variation in the fatty acid composition of phospholipids is particularly important during the process of adaptation to temperature changes. Generally, there is an increase in the proportion of PUFAs and monounsaturated fatty acids (MUFAs) and a reduction of saturated fatty acids (SFA) in membrane phospholipids. The increase in PUFAs allows the membrane of cells to continue functioning properly. In this regard, the rich constitution of bioactive compounds of Aurantiochytrium sp. may be associated with the beneficial effects observed in our study, particularly the increase in shrimp resistance against viral infection at low temperatures, and more specifically, the supplementation levels of 3 and 4 percent.
However, when the shrimp were subjected to thermal stress by raising the temperature, the animals supplemented with 3 percent of Aurantiochytrium sp. showed high mortality, matching the values of the control (0 percent). This low tolerance to thermal oscillation of animals from 3 percent treatment was not fully understood, requiring further investigations.
Perspectives
Our results showed that the dietary inclusion of Aurantiochytrium sp. meal had no effect on the growth performance of P. vannamei cultivated at suboptimal temperatures. However, the 4 percent dose resulted in lower mortality of shrimp after the viral challenge with WSSV associated with temperature stress.
Now that you've reached the end of the article ...
… please consider supporting GSA’s mission to advance responsible seafood practices through education, advocacy and third-party assurances. The Advocate aims to document the evolution of responsible seafood practices and share the expansive knowledge of our vast network of contributors.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year.
Not a GSA member? Join us.
Author
-
Delano Días Schleder
Corresponding autor
Instituto Federal Catarinense, Campus Araquari, Araquari 89245-000, Brazil
Tagged With
Related Posts

Aquafeeds
Assessment of microalgal biomass as a fish oil replacement in Atlantic salmon diets
The inclusion of a microalgal biomass in salmon feeds shows potential use throughout the whole production cycle from parr to harvest size.
Aquafeeds
Effects of dietary supplementation with the marine microalgae Aurantiochytrium sp. on silver pompano
Evaluating Aurantiochytrium sp. diet supplementation on silver pompano shows enhanced fish growth, physiological and biochemical indexes.

Health & Welfare
An update on vibriosis, the major bacterial disease shrimp farmers face
Vibrios are undoubtedly the foremost cause of bacterial disease outbreaks in farmed shrimp, but the significant role of stressors is often ignored.

Health & Welfare
Influence of stressors on shrimp susceptibility to White Spot Disease, Part 1
Study stresses the importance of understanding the impact of environmental stressors on farmed shrimp contracting White Spot Disease.



