Results suggest protection for fish, alternative approach for testing vaccines

Tilapia farming in Zambia is a relatively young but rapidly growing industry. Although the industry started in the 1990s, it was in 2010 when cage-based commercial farming intensified on Lake Kariba and production expanded. Annual aquaculture production presently stands around 30,000 metric tons (MT).
But as with intensive fish farming elsewhere, the increase in fish production in Lake Kariba soon faced disease problems. Outbreaks of the pathogenic bacterium Lactococcus garvieaehave been experienced since production expanded early in this decade.
The bacterium L. garvieae is well-known for infecting and causing disease in other finfishes like rainbow trout yellowtail. Clinical signs include exophthalmia, conjunctivitis, melanosis, erratic swimming, anorexia, internal hemorrhage and congestion of blood vessels, peritonitis, meningoencephalitis and septicaemia.
In tilapia, L. garvieae infections cause an emerging disease that became of major importance during the last decade. These infections are most severe when water temperatures are above 20 degrees-C. Economic losses occur as a result of mortalities (high or low), quality downgrading fish due to unsightly skin lesions and reduced growth rate. No protective commercial vaccines for tilapia are available on the market at the moment.
This article – adapted and summarized from the original – reports on a study to develop a whole bacterial cell, autogenous, oil-based vaccine for the protection of Nile tilapia (Oreochromis niloticus) against L. garvieae infections. Autogenous vaccines are custom-made and are produced on a small to medium scale and are based on pathogens isolated from the farm where they are to be used. They have the advantage of being less amenable to rigorous regulations applicable to commercial vaccines and allow for more rapid availability without complete and comprehensive characterization in the face of an outbreak.
Study setup
A total of 460 healthy Nile tilapia (mean weight 41.5 ± 16.5 grams) were purchased from Palabana Fisheries – a commercial fish farm with no previous history of disease outbreaks and located in the Chirundu District in southeastern Zambia – and transported to the wet lab at the School of Veterinary Medicine of the University of Zambia. The fish were kept in 500-liter aerated tanks with flow-through de-chlorinated water, and allowed to acclimatize for 10 days prior to starting the experiment. The tilapia were fed daily with commercial pellets at a rate of 3 percent body weight. During the trial, water temperature averaged 20 ±2 degrees-C, mean daily dissolved oxygen was 7.9 ± 2 mg/L and pH was 7 ± 0.2.
For antigens and vaccine formulation, the L. garvieae used was previously isolated from a diseased fish at a farm on Lake Kariba. The bacteria were propagated, incubated, centrifuged and then inactivated. The vaccine was formulated using 109 CFU (colony forming units)/mL as a water-in-oil emulsion using the ISA 763 VG adjuvant (a pharmacological or immunological agent that improves the immune response of a vaccine) from Seppic, France, and according to the manufacturer’s guidelines. The adjuvant-only group was prepared in the same way but without bacteria. The preparations were then stored at 4 degrees-C until used.
The 450 fish were divided into three groups [Control (in phosphate-buffered saline, or PBS), Adjuvant and Vaccine], each with 150 individuals. The control fish were injected with PBS, the Adjuvant group were injected with adjuvant only, and the Vaccine group with the L. garvieae vaccine. The total number of fish per group was 150 individuals. Each group was further split into two replicates, one for observation (surveillance) and the other for sampling. For sampling, each group was placed in a separate tank (A-C), each containing 90 individuals. The rest of the fish were pooled together in tank D (surveillance), containing 60 control, 60 vaccinated and 60 adjuvant-only groups all mixed together (Fig. 1). The fish in tank D were marked by clipping of the dorsal fin, caudal fin or left unclipped to differentiate between groups. All fish were injected intraperitoneally with 0.1 mL of vaccine, adjuvant-only or PBS.
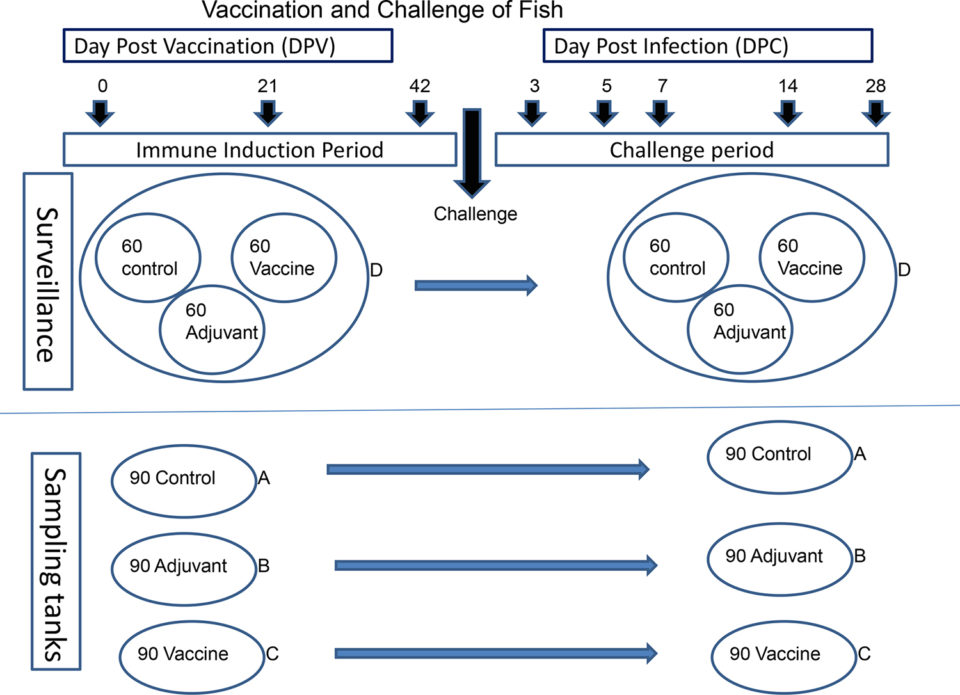
Following vaccination, all fish in the four tanks (A, B, C and D) were allowed a period of six weeks for immune induction (Fig. 1). On day 43 post vaccination (dpv), the fish were challenged by intraperitoneal injection of 0.1 mL of L. garvieae suspension (9.6 x105 CFU bacteria/fish). Monitoring was done for 28 days post-challenge (dpc), during which clinical signs were recorded and sampling for bacterial re-isolation was done.
For detailed information on antigens and vaccine formulation; fish vaccination; the challenge experiment; sample collection; various laboratory tests and procedures used; and statistical analyses, refer to the original publication.
Results and discussion
In the experimental groups, clinical signs of disease were observed mostly in the control (PBS) and adjuvant only groups. The most common sign was ocular opacity, uni- or bilateral, with or without exophthalmia (bulging eyes). The frequency was highest in the control group followed by the adjuvant only group. In the former, fish with clinical signs were first observed at the days post challenge (dpc) and culminated on 5 dpc.
In the adjuvant group, the first onset of clinical signs was on 5 dpc followed by 7 dpc. In the vaccinated group, only two fish showed clinical signs, one at 3 dpc, likely due to physical injury unrelated to the pathogen challenge, and another one at 14 dpc, this time with corneal opacity. No mortalities were observed in any of the groups.
Our findings suggest that the mechanism for fish protection is achieved is likely via an antibody mediated response. No clinical signs or post-mortem changes were observed in the vaccinated group nor was L. garvieae re-isolated from any of the tissues (except from the spleen of one fish) at any time point following challenge. In contrast, clinical signs and post-mortem changes were observed in the adjuvant only and unvaccinated control groups during the first 14 days following the challenge. Furthermore, significantly more L. garvieae was re-isolated from the control and adjuvant only groups in this study during the first seven days.
No bacteria was re-isolated from any fish in any group from 14 dpc. The reason for this is yet to be investigated, but L. garvieae under experimental conditions has been shown to induce acute infections, typically within 10 days post infection. Fish that do not succumb within this time recover from the infection, and it is not unlikely that they can clear the bacteria with time. The low prevalence of infection in our study contrast with reports from other researchers where significant mortalities were observed following challenge of tilapia with L. garvieae.
We used immunohistochemistry [the application of immunostaining, the process of selectively identifying antigens (proteins) in cells of a tissue section by exploiting the principle of antibodies binding specifically to antigens in biological tissues] techniques as an additional method to demonstrate, in situ, the presence of L. garvieae in different organs in the fish. This method detects both viable bacteria at the time of sampling, and also unviable/inactivated bacteria, including antigens in the vaccine used. It is therefore not surprising that L. garvieae was demonstrated in the vaccinated group where no corresponding bacteria were re-isolated., and consistent with previous reports.
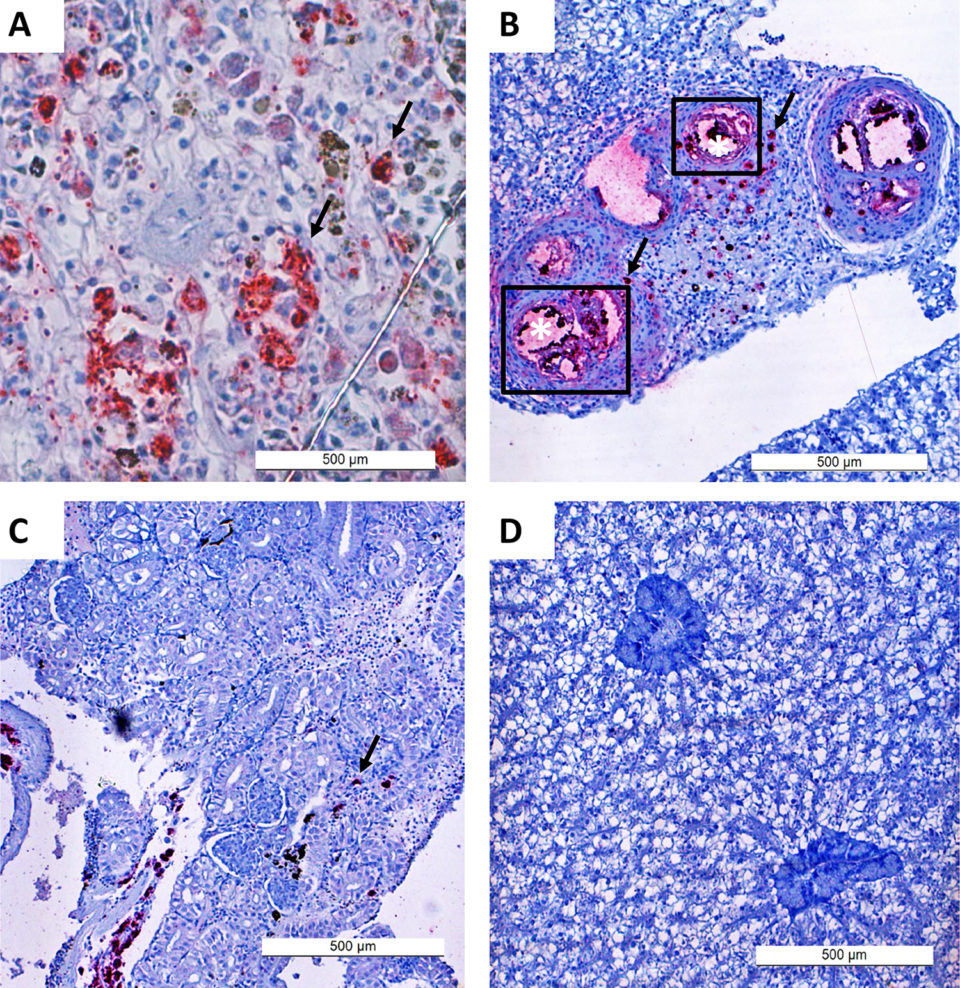
In our study, L. garvieae infection establishment in tilapia progressed very rapidly, peaking within three to five days and was consistent with the findings of other scientists. The distribution of L. garvieae in different tissues (based on the trends and frequency of infected organs over time) we observed from bacterial re-isolation and immunohistochemistry in the adjuvant and control groups suggests that bacterial localization first occurs in the liver, kidney and spleen before spreading to the brain.
Nile tilapia immunized with oil-based L. garvieae vaccine in our study produced significantly higher amounts of antibodies than the control or adjuvant only groups by 21 days post vaccination. This trend continued until three days post challenge, when the antibody titers [how much specific antibody an organism has produced] dropped sharply. The high antibody titers in the vaccinated group at the point of challenge and the absence of bacteria in tissues of this group – as demonstrated by lack of bacterial re-isolation – suggest that antibodies play a significant role in the protection of the fish against infection. This agrees with the mechanism of action of oil-adjuvanted vaccines and other known protective mechanisms against extracellular pathogens.
Perspectives
The primary objective of this study was to develop a protective, oil-based, autogenous vaccine for the protection of tilapia on Lake Kariba. Our findings suggest that a whole bacterial cell autovaccine we developed can protect Nile tilapia against L. garvieae infection. The vaccinated group in our study was significantly more protected than the adjuvant only or the control groups. These findings support the reports of other researchers that oil-adjuvanted vaccines can induce protection of tilapia against infection with L. garvieae.
Also, our results provide an alternative approach for testing vaccines that does not involve mortality, in line with fish welfare and the reduction in fish suffering. Recommend additional studies to confirm our findings.
References available from original publication.
Now that you've reached the end of the article ...
… please consider supporting GSA’s mission to advance responsible seafood practices through education, advocacy and third-party assurances. The Advocate aims to document the evolution of responsible seafood practices and share the expansive knowledge of our vast network of contributors.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year.
Not a GSA member? Join us.
Authors
-
Patricia Bwalya, Ph.D.
Faculty of Veterinary Medicine
Norwegian University of Life Sciences, Oslo, Norway; and
Samora Machel School of Veterinary Medicine, University of Zambia, Lusaka, Zambia; and
Department of Veterinary and Livestock Services
Ministry of Fisheries and Livestock
Lusaka, Zambia -
Bernard M. Hang’ombe, Ph.D.
Samora Machel School of Veterinary Medicine
University of Zambia
Lusaka, Zambia -
Amr A. Gamil, DVM
Faculty of Veterinary Medicine
Norwegian University of Life Sciences
Oslo, Norway -
Hetron M. Munang'andu, DVM, Ph.D.
Faculty of Veterinary Medicine
Norwegian University of Life Sciences
Oslo, Norway -
Øystein Evensen, DVM, Ph.D.
Faculty of Veterinary Medicine
Norwegian University of Life Sciences
Oslo, Norway -
Stephen Mutoloki, Ph.D.
Corresponding author
Faculty of Veterinary Medicine
Norwegian University of Life Sciences
Oslo, Norway
Tagged With
Related Posts

Innovation & Investment
AquaGen CEO: Genomics are transforming aquaculture
The CEO of AquaGen knew that the Norwegian research group’s work in genomics was key to the salmon industry’s future. And that was before she even worked there.
Health & Welfare
Developing live bacterial vaccines by selecting resistance to antibacterials
As wide use of antibiotics has led to antibiotic resistance in fish pathogens, vaccines present an alternative control method to prevent bacterial diseases.

Health & Welfare
Pellet-based oral vaccine holds promise for VNN protection
An oral vaccine delivered via fish feed to defend against Viral Nervous Necrosis could be ready for market soon if everything goes according to plan for startup VakSea.

Intelligence
10 takeaways from GOAL 2019 in Chennai, India
The Global Aquaculture Alliance held its GOAL conference in Chennai, India, and recruited a host of experts in various fields to share their expertise.


