Research conducted at Aquatic Medicine Laboratory of the Virginia-Maryland Regional College of Veterinary Medicine
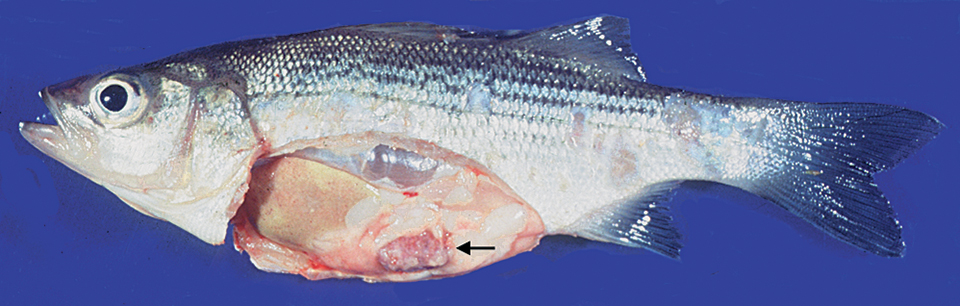
Wild and cultured fish around the world are commonly affected by piscine mycobacteriosis. In recent years, outbreaks of this disease in the Mediterranean Sea and Chesapeake Bay in the United States have had severe ecological impacts on feral fish populations. Case reports also testify that mycobacteriosis is an omnipresent problem in public aquariums and foodfish, baitfish, and ornamental fish production facilities.
Piscine mycobacteriosis
Piscine mycobacteriosis can be caused by a number of bacteria of the genus Mycobacterium, but M. marinum is most often the primary agent. Infections often manifest as either an acute or chronic disease that causes a variety of clinical signs from lethargy and emaciation to systemic infection, granuloma formation, and death.
Unfortunately, chemotherapeutic agents and other biologics are not available for treatment or prevention of this disease, and most veterinarians and fish health professionals recommend the destruction of affected or exposed populations. Furthermore, piscine mycobacteriosis can be transmitted from fish to humans, causing “fish handler’s disease” or “swimming pool granuloma” and warranting long-term medical treatment. Given the ecological, economic, and human-health implications, methods to prevent this disease deserve careful consideration.
Vaccine research
The Aquatic Medicine Laboratory of the Virginia-Maryland Regional College of Veterinary Medicine in Blacksburg, Virginia, USA, has undertaken work to develop a vaccine to prevent piscine mycobacteriosis. Recently, this research has centered on creating a DNA vaccine for the disease.
DNA vaccines have been widely studied for mammalian diseases, but are relatively new to the fish world. The technology is based on introducing DNA that encodes an antigen into animal tissue, which itself then expresses that antigen. As a result, an immune response may be generated against the target antigen, which in turn helps protect the animal against infection.
In mammals, this type of vaccine stimulates the type of immunity required to prevent the development of mammalian mycobacteriosis. The authors hypothesized that similar effects would be stimulated in fish.
The DNA vaccine developed in the Aquatic Medicine Laboratory encodes a protein antigen from M. marinum that is vital to the development of mycobacteriosis. Hybrid striped bass (Morone saxatilis × M. chrysops), were utilized as the research model because they are known to be highly susceptible to mycobacteriosis. When injected with the vaccine in muscle or the abdominal cavity, the fish generated significant immune responses, including increased specific antibody production and enhanced lymphocyte activities.
More importantly, after challenge exposure to high doses of live M. marinum on 90 or 120 days after injection, the vaccinated fish exhibited lower splenic bacterial counts, reduced granuloma formation, and mortality rates below those of unvaccinated fish. These findings indicated the DNA vaccine can be both immunogenic and protective against infection, and is a promising candidate for use in fish. Further evaluation of the efficacy, safety, and production costs will help determine the full potential of this piscine mycobacteriosis vaccine.
Effective prevention
Typically, a significant number of fish in an aquaculture or hatchery facility succumb to disease propagated by factors common in aquaculture operations, including overcrowding, improper nutrition, poor water quality, and inadequate biosecurity. However, vaccines can be used to help protect fish against disease.
An effective vaccine may increase the cost of production by a few cents per fish, but should decrease disease-related mortalities and allow more fish to be stocked or reach market. An alternative is to treat sick fish with therapeutics, but the control of disease with therapeutics is historically more expensive than prevention with vaccination.
Approved fish vaccines
Traditionally, killed and live attenuated vaccines have been developed for use in fish. A number of them are approved for use in the United States, Canada, Europe, or Asia against diseases such as furunculosis, enteric red mouth, columnaris, and enteric septicemia of catfish. The websites “Currently Available Biologics for Fish,” http://www.aphis.usda.gov/vs/cvb/aqualicenses.htm; and “Worldwide Aquaculture Drug and Vaccine Registration Progress,” http://ag.ansc.purdue.edu/aquanic/jsa/aquadrugs/publications/world_drug_progress_9-20-99.htm; list approved vaccines. Please refer to national regulatory authorities before use, because vaccine approval status and availability may change.
Evolving vaccine technology
Currently, several modern vaccine technologies, such as DNA, recombinant, and genetically attenuated vaccines, are being evaluated for use in fish. These vaccines contain whole pathogens or pathogen components that have been altered and rendered noninfectious. These may provide advantages over more traditional vaccines because they often exhibit improved competence for preventing infectious diseases like mycobacteriosis.
Development of these vaccines is still in the early stages, but continued research offers an optimistic future for their utility. Expansion of the available vaccine repertoire will allow veterinarians, fish health professionals, aquaculturists, and pet fish owners to better protect wild and cultured fish from disease.
(Editor’s Note: This article was originally published in the October 2003 print edition of the Global Aquaculture Advocate.)
Now that you've reached the end of the article ...
… please consider supporting GSA’s mission to advance responsible seafood practices through education, advocacy and third-party assurances. The Advocate aims to document the evolution of responsible seafood practices and share the expansive knowledge of our vast network of contributors.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year.
Not a GSA member? Join us.
Authors
-
David J. Pasnik, DVM, M.S.
Aquatic Medicine Laboratory
Virginia-Maryland Regional College of Veterinary Medicine
Virginia Polytechnic Institute and State University
Blacksburg, Virginia 24061 USA -
Stephen A. Smith, DVM., Ph.D.
Aquatic Medicine Laboratory
Virginia-Maryland Regional College of Veterinary Medicine
Virginia Polytechnic Institute and State University
Blacksburg, Virginia 24061 USA
Tagged With
Related Posts
Health & Welfare
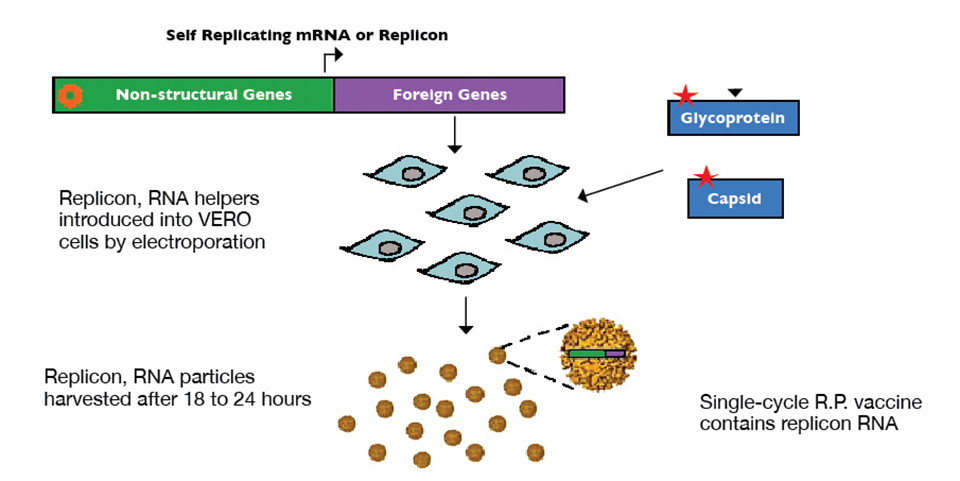
Alphavirus replicon particles potential method for WSSV vaccination of white shrimp
A study demonstrated that VP19 and VP28 white spot syndrome virus envelope proteins expressed by replicon particles provided protection against mortality due to WSSV in shrimp.

Innovation & Investment
AquaGen CEO: Genomics are transforming aquaculture
The CEO of AquaGen knew that the Norwegian research group’s work in genomics was key to the salmon industry’s future. And that was before she even worked there.
Health & Welfare
Developing live bacterial vaccines by selecting resistance to antibacterials
As wide use of antibiotics has led to antibiotic resistance in fish pathogens, vaccines present an alternative control method to prevent bacterial diseases.
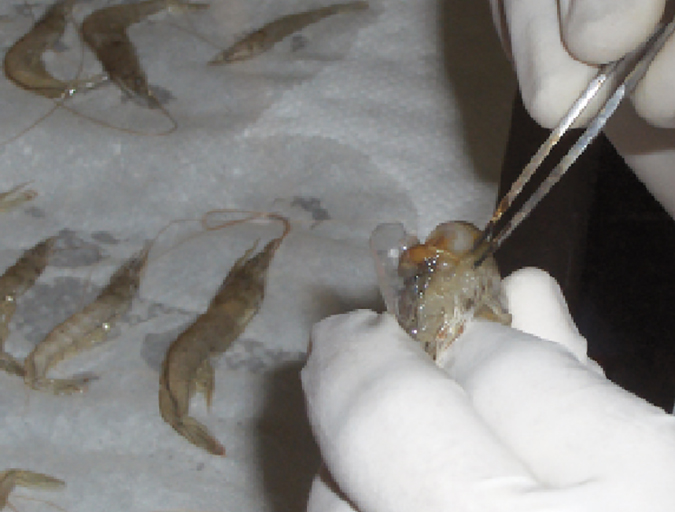
Health & Welfare
Double-stranded RNA against WSSV genes provides antiviral protection in shrimp
Silencing genes in white spot syndrome virus (WSSV) with critical roles in replication could provide a strong antiviral effect and thus reduce shrimp mortality. The authors therefore established a study to evaluate the antiviral efficacy of double-stranded (ds)RNA against non-structural WSSV genes.



