How aqueous brine solution works and other freezing techniques for processing plants

Foods in general – seafood in particular, and especially shrimp – are subjected to biological and biochemical phenomena such as respiration and biochemical reactions catalyzed by enzymes (oxidation, proteolysis, lipolysis) that deteriorate them. This alteration of food is also due to the action of microorganisms.
The prevention of microbial contamination by the application of the concepts of hygiene and cleaning is a necessary principle before considering any methods of eradication or containment of the microorganisms and their enzymatic activities. It is, therefore, essential to use appropriate conservation techniques to handle these products regardless of the location and the season.
There are several food preservation technologies, including preservation by fermentation (lactic like for cheese, and alcoholic like for wine); decreased water activity (dehydration, salting); thermal treatment (sterilization or freezing); and other means of conservation like ionizing radiation, treatment with bactericidal gases, storage under modified atmospheres, high-pressure treatment, and others.
Use of cold temperature
Cold temperature has the effect of slowing or inhibiting the phenomena that alter foodstuffs by acting on the microorganisms and on the course of biochemical reactions. The application of cold temperature to foodstuffs can be done through refrigeration, and by freezing and deep freezing, with several degrees of efficiency.
Refrigeration is a process where the temperature of the product is lowered so that it is close to the temperature of melting ice. This slows down the development of micro-organisms and the biological processes but does not stop them. In general, during refrigeration, a portion of the water in the product evaporates.
During freezing and deep freezing, the water in the product solidifies to ice. At temperatures below -18 degrees-C, development of all microorganisms and biochemical changes are stopped, which results in frozen products having shelf lives from several months to more than one year. The quality of the product depends on the quickness to lower the temperature of the product to any point below -18 degrees-C, to crystallize all the water contained in the product.

The freezing of foodstuffs can cause problems related to the expansion of the water volume during the transition from the liquid state to the solid state. This mostly involves cell wall breakage and the release of intracellular fluid during the thawing (exudation) process. The damage principally depends on the size of the ice crystals formed within the cells. During slow freezing, there is a progressive growth of extracellular ice crystals that causes the cells to be emptied by osmosis, causing significant damage. In contrast, during rapid freezing, small intracellular and extracellular crystals are formed with minimal damage.
Compared to other preservation methods – such as dehydration, salting, cooking, pasteurization, sterilization and others – that significantly alter the characteristics of the products, freezing is a preservation technique that, when properly done, preserves the food’s original qualities with minimal alteration and maximum freshness.
Different techniques used to freeze shrimp
To freeze a product, it is necessary to install a thermic exchange between a warm product (the shrimp) and a dynamic cold fluid (Fig. 1). In contact with the “hot” product, the “cold” fluid is heated by lowering the temperature of the product. If the cold fluid remained statically in contact with the product, a thermal equilibrium would be reached that would be midway between the temperatures of the fluid and of the product, which would not be satisfactory. It is, therefore, necessary to maintain the temperature of the fluid by circulating it between the product and a refrigeration system that will maintain its low temperature. The efficiency of the system will depend of the heat transfer coefficient of the cold fluid.
Several techniques are used to freeze shrimp:
Air blast freezers: The cold fluid is the air circulating between an evaporator on which the temperature of the air is lowered and the shelves with shrimp blocks, where the product “absorbs” the frigories (negative calories) and heat the fluid (the air) which is chilled by passing back into the evaporator. The spiral freezers are using the same principle with the freezing airflow.
Contact plate freezer: In this case the cold fluid circulate in the plates and freeze them. This system needs a contact between the box and the plates. It is efficient for the block in water which transmit the temperature but not so much with the semi block without water
Brine freezers technology: discussed in this article.
Other techniques are also used, but they are still very confidential because the operational costs are too high as they involve cryogenics using liquid nitrogen, or are not as well suited to freeze thick boxes as the impingement freezers, which are very efficient for IQF but not for boxes. The impingement freezers are adequate for thin layers of product, but when they are too thick, the process does not work
Table 1 presents the different types of processes used for refrigeration and freezing and their main applications. It also shows the maximum values of heat transfer coefficients achieved in practice for comparing the performance of each type of technology.
Lucien-Brun, brine, Table 1
| Process | Refrigeration (T>0 degrees-C) | Freezing (T<0 degrees-C) | Heat transfer coefficients [W/(m2 x K)] |
|---|---|---|---|
| By air (blast freezer, others) | Fruits, vegetables, meat, seafood | All products | 20 to 50 (air blast tunnel); 60 (fluidized bed freezing) |
| By immersion (water or aqueous solution) | Poultry, fruits and vegetables, fish and crustaceans | Fish, crustaceans | 900 |
| Cryogenics (liquid nitrogen or carbon dioxide) | – | Flash freezing, crusting products | 100 |
| By contact | Liquid products (milk, others), fish (flake ice) | Milk, fruit juice, fish fillets, vegetable purees | 100 |
The heat transfer coefficients of water or aqueous solutions are in the order of 10 to 50 times higher than for air, and may reach up to 900W/m2 x degrees-K. These solutions have interesting characteristics from the point of view of processing speed.
Immersion of the products to be frozen in aqueous solutions is one of the fastest cooling techniques, but is currently limited to a few specific applications. This is a technique that could be extended to other areas, but there are still needs for improvement. The brine freezing technique, due to its high heat transfer coefficient, is also a method that requires much less energy than traditional methods.
Immersion in chilled brine (aqueous solution) is commonly used by many industrial cookers to chill the shrimp after cooking. For several years now this technique has also been commonplace in shrimp packing plants in Mexico and widely used to deep-freeze raw, farmed shrimp; to temporarily freeze shrimp before value-added processing; or to freeze shrimp for markets that demand maximum yield.
Because freezing in brine is very rapid, the crystals formed inside the shrimp tissues are very small and only cause very small ruptures of cell membranes. Consequently, there is very little liquid left after defrosting, and therefore a significantly lower loss than that observed from the “traditional” freezing techniques like air blast tunnels or contact plates freezers.
And the penetration of salt into the superficial flesh results in a better cooking yield when compared to the other freezing techniques. Moreover, the shrimp texture remains very like that of fresh shrimp, and just as the best taste is a very desirable characteristic, a good texture is another quality criterion that is very well appreciated. This is why this technique is highly valued by customers who have access to the most demanding markets.
How brine freezing works
Table 1 shows that the most efficient temperature transfer results from immersion in an aqueous solution. An issue is that water become solid (ice) at 0 degrees-C, and a temperature of minus-18 degrees-C is needed to freeze the product. The addition of salt (sodium chloride) to the water allows lowering the temperature of water freezing into ice, as a eutectic system.
A eutectic system is a mixture of chemical compounds or elements that has a single chemical composition that solidifies at a lower temperature than any other composition. This composition is known as the eutectic composition and the temperature is known as the eutectic (the lowest temperature of solidification for a mixture of materials) temperature.
When the salt (NaCl; Na+, Cl–) comes in contact with the ice, the ions arrange themselves around the water molecules that are polarized (H2δ+ Oδ) and form a compound (H2O) (NaCl). This rearrangement requires only small movements of atoms and is therefore a solid phase. Where the exact proportions are observed (about 22 NaCl, at a density of 1.170 Kg/m3), the mixture behaves as a pure product, described as “eutectic.” The melting of this eutectic NaCl–H2O mixture is about -21.2 degrees-C (Table 2).
The diagram of phases (Fig. 2) illustrates the melting temperature of the mixture depending on the water-salt ratio.
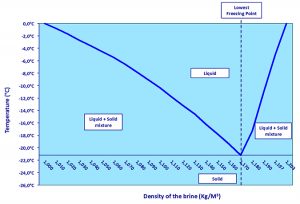
Thus, for salt levels lower than the eutectic proportion we get a water and eutectic mixture that melts at a temperature above -21.2 degrees-C. And for higher salt levels the result is the eutectic mixture that solidifies at a lower temperature
Rearrangement of the salt and water eutectic mixture can only happen at the ice surface, the points of contact between the ice crystals and the salt.
When a layer of eutectic melts is formed (if the temperature is higher than -21.2 degrees-C) as the salt is supersaturated, it dissolves in the eutectic melt and can react with the ice that lies beneath the liquid film. This phenomenon spreads until there is lack of water or salt to form a new eutectic.
Components of a brine for freezing shrimp
Brine freezing of shrimp involves two steps. The first one is to decrease the temperature of shrimp from positive to a range of minus-8 to minus-12 degrees-C with the brine.
The second step involves reaching the deep-freezing temperature of -18 degrees-C in an air blast tunnel. The latter is a very fast process that takes between 20 minutes and two hours, depending on the tunnel design. Because the product is already at a negative temperature, the energy needed to bring it to -18 degrees-C is much lower than if a product at positive temperature is directly frozen in the tunnel. And it shortens the duration of the second phase of freezing. The components of the brine used for the first step are water, salt, sugar and additives.
Water: Water is the solvent of the solution; freshwater must be used that is drinkable and tasteless
Salt: The salt used is common sea salt (sodium chloride, NaCl). In addition to affecting the product’s organoleptic quality (salting) and also its microbiological quality (bacteriostatic effect), salt has the property of lowering the freezing point some water. Indeed, the freezing point of pure water is 0 degrees-C, and this point will be moved to negative temperatures after the dilution of a defined quantity of salt.
Table 2 shows the thermo-physical properties of a sodium chloride aqueous solution and the freezing point depending on the NaCl concentration. The dissolution of the salt in water is expressed by the ionic dissociation of the NaCl molecule releasing free Na+ and Cl– ions in water. A common issue involves confusing the salt concentration in the water and the salt concentration in the brine; to avoid it, the easiest way is to consider only the density when preparing new brine.
Lucien-Brun, brine, Table 2
| Mass of salt (%) per 100 kg of water | Mass of salt (%) per 100 kg of brine | Density at 15 degrees-C; Baumé degrees | Density at 15 degrees-C; Density (Kg/dm3) | Freezing point (degrees-C) |
|---|---|---|---|---|
| 0.1 | 0.1 | 0.1 | 1.000 | 0.0 |
| 1.5 | 1.5 | 1.6 | 1.010 | -0.8 |
| 3.0 | 2.9 | 3.0 | 1.020 | -1.7 |
| 4.5 | 4.3 | 4.3 | 1.030 | -2.7 |
| 5.9 | 5.6 | 5.7 | 1.040 | -3.6 |
| 7.5 | 7.0 | 7.0 | 1.050 | -4.6 |
| 9.0 | 8.3 | 8.3 | 1.060 | -5.5 |
| 10.6 | 9.6 | 9.6 | 1.070 | -6.6 |
| 12.3 | 11.0 | 10.8 | 1.080 | -7.8 |
| 14.0 | 12.3 | 12.0 | 1.090 | -9.1 |
| 15.7 | 13.6 | 13.2 | 1.100 | -10.4 |
| 17.5 | 14.9 | 14.4 | 1.110 | -11.8 |
| 19.3 | 16.2 | 15.6 | 1.120 | -13.2 |
| 21.2 | 17.5 | 16.7 | 1.130 | -14.6 |
| 23.1 | 18.8 | 17.8 | 1.140 | -16.2 |
| 25.0 | 20.0 | 18.9 | 1.150 | -17.8 |
| 26.9 | 21.2 | 20.0 | 1.160 | -19.4 |
| 29.0 | 22.4 | 21.1 | 1.170 | -21.2 |
| 31.1 | 23.7 | 22.1 | 1.180 | -17.3 |
| 33.1 | 24.9 | 23.1 | 1.190 | -11.1 |
| 35.3 | 26.1 | 24.2 | 1.200 | -2.7 |
| 35.7 | 26.3 | 24.4 | 1.203 | 0.0 |
Sugar is used in the brine mainly for its coating properties to avoid a strong salting of the shrimp. The sugar used is generally common cane sugar, mostly sucrose, a disaccharide combination of the monosaccharides glucose and fructose. It is a non-reducing sugar with a high molar mass of 342 g/mol, much higher than for salt (NaCl), which is only 58.5 g/mol.
Once dissolved in the brine, the sugar gives it a certain viscosity, which is not the case for brine made only with salt and water (sugar makes products sticky, not the salt).
Thus, because of its high molecular viscosity and also its molecular weight relative to that of the salt, the sugar will form a barrier or a protective layer reducing the penetration of the salt into the product. The sugar is used as a “coater” to prevent the shrimp from being too salty. It is for this reason that peeled shrimp can also be frozen in brine including sugar, but not in brine made only of salt and water. This layer of surface protection will give the shrimp a shiny appearance that is appreciated by consumers, and which cannot be obtained with brine consisting solely of salt and water.

Another advantage of using sugar is that it facilitates remolding the product if needed; the less sugar there is, the more the shrimp stick firmly on the walls of the mold (forming template) and are difficult to unmold. Furthermore, sugar has a synergy with salt for lowering the freezing (solidification) temperature of the brine. Adding sugar makes it possible to lower even further the freezing temperature of the brine.
Additives include chlorine and antifoaming products. If necessary, chlorine can be used to make potable the water used to prepare the brine. The concentration used is based on the quality of the local water, and up to 25ppm can be used to reduce bacterial contamination. Antifoaming products can be used to prevent or break up any brine foaming during return to the tank in the freezing process.
Silicone-based antifoam agents are commonly used; these are food additives presenting no risk for the products to be frozen. Their concentrations are based on the objectives desired for product temperature, refrigeration or deep-freezing; for the freezing rate; and eventually according to the customer specifications.
The main risk when the brine is inadequately prepared or managed is system icing, which requires a complete stoppage of production activities to increase the temperature and allow the brine to melt. The appearance of solid ice is mainly due to the decrease in salt concentration. However, the use of sugar would reduce the risk of icing.
When increasing the sugar concentrations of the brine and decreasing the tunnel temperature during the second phase, the temperature of the shrimp coming out of the tunnel can reach -22 to -24 degrees-C. This is not appropriate and is even dangerous, and in fact, the cold chambers where clients store shrimp products are generally regulated at the legal temperature of -18 degrees-C.
Indeed, the little amount of brine remaining on the shrimp will not be frozen (solidified in ice) at -18 degrees-C. This brine will remain in the liquid phase, creating two issues. First, it will drip a little at a time and after a few weeks, the cartons where the processed shrimp are packed will develop a damp appearance.
Second, and worse, because the brine remains in a liquid phase, all biological mechanisms for the formation of black spots will not be prevented and, despite treatment with sodium metabisulfite, the black spots of melanosis may appear in those areas that have higher moisture. The result is that these shrimp will not be accepted in many markets.
Editor’s note: Part 2 will discuss the different brine freezing systems, and brine management.
Author
-

Hervé Lucien-Brun
Aquaculture & Qualite
9 Rue Poupinel
F-78730 Saint Arnoult en Yvelines, France
Tagged With
Related Posts

Intelligence
Critical decisions for shrimp harvesting and packing, Part 1
Harvesting a crop of shrimp is a critical step for any shrimp farming venture. Several months of efforts and resources to properly raise a quality crop have already been invested, and this quality must be preserved. The decision to harvest involves consideration of various factors.

Intelligence
Critical decisions for shrimp harvesting and packing, Part 2
The decision to harvest involves consideration of various factors, including market prices, customers’ need, biomass and condition of the animals and others. Several steps are involved, most aimed at maintaining maximum quality of the animals as they are harvested, sacrificed, treated and transported to the plant and processed and packed.

Intelligence
Critical decisions for shrimp harvesting and packing, Part 3
In this final installment of Hervé Lucien-Brun’s comprehensive three-part series, the author details the final steps in the process of taking farmed shrimp to the marketplace. Here we take a look at the proper protocols for receiving at the processing plant, weighing, grading and freezing.

Health & Welfare
Saudi Arabia developing effective farmed shrimp biosecurity strategy
Biosecurity strategies adopted by the shrimp industry in the Kingdom of Saudi Arabia have resulted in historical production in 2015 and a similar forecast for 2016, and are a significant step towards the long-term goal of sustainable aquaculture.

