Determining in vitro anti-inflammatory and immunogenic capacity

The use of insect meal, mainly derived from larvae of black soldier fly (BSF, Hermetia illucens) as an aquafeed ingredient is under investigation while being increasingly adopted by the aquafeed industry. Most research has focused on the potential of insect meal as an alternative protein ingredient for several fish species – including rainbow trout, salmon, carp, sea bass and catfish – while only a few studies have investigated the immunological benefits of insect meal.
Here we present the results of a project aimed at investigating the in vitro immunogenic (or pro-inflammatory) and anti-inflammatory capacity of BSF organic black soldier fly meal compared to other commercially available ingredients.
Study setup
Ingredients tested
Descriptions of the ingredients investigated throughout this project are included in Table 1. All samples were dissolved in water, mixed at room temperature for one hour, and centrifuged to separate soluble (supernatant) from insoluble (pellet) material. The supernatant, containing soluble components, was 0.22 µm filtered and stored at minus-20 degrees-C until needed for cell assays.
Rombenso, BSF, Table 1
| Ingredients tested | Abbreviation | Concentrations tested |
|---|
Ingredients tested | Abbreviation | Concentrations tested |
|---|---|---|
| Whole black soldier fly organic insect meal | BSF Pre | 20 mg/mL |
| Expeller-pressed black soldier fly organic insect meal–low fat | BST Post | 20 mg/mL |
| Fishmeal | – | 20 mg/mL |
| Cricket powder | – | 20 mg/mL |
| Quercetin | – | 100 µM |
Cell-based assays
The RAW264.7 mouse macrophage cell line was grown in vitro to produce nitric oxide (NO), a signaling molecule that plays a vital role in the pathogenesis of inflammation. Stimulation of NO production represents immunogenic or pro-inflammatory responses while decreased NO production suggests anti-inflammatory activity. RAW264.7 mouse macrophage cells were grown to produce two different cell-based assays.
Meta-analysis of the effects of black soldier fly meal on fish growth
Immunogenic (pro-inflammatory) capacity in vitro

To stimulate the production of NO and observe any immunogenic (or pro-inflammatory) responses, cells were treated with bacterial lipopolysaccharide (LPS) or the test ingredients. To ensure that any treatment effect was not related to toxicity (that could result in false positives), cell viability was measured in the assay in response to all project samples and ranged from 106–124 percent (expressed as a percentage compared to untreated cells).
The following methodology was followed:
- 7 cells were cultured in flasks and maintained at 37 degrees-C with 5 percent CO2 in growth media (DMEM, 10 percent fetal bovine serum).
- Cells were cultured until approximately 90 percent confluent before being collected and counted.
- 6×1057 cells/mL were plated into clear 96-well plates and cultured for two days in the presence of water extracts prepared from test samples (see Table 1) diluted in growth media.
- After two days, cell culture media was transferred to black 96 well plates for nitrite production (i.e., detection of nitric oxide) using DAN (2,3-Diaminonaphtaline).
- After 10 minutes, fluorescence was measured (Ex 360 nm; Em 430 nm) and plotted against a nitrite standard curve.
- Results were further extrapolated using an LPS-nitrite production standard curve.
- The cells remaining in the clear plates were washed before cell viability was measured at 490 nm, using CellTiter96 Aqueous One Solution Cell Proliferation Assay (Promega).
Anti-inflammatory capacity in vitro
In this assay, cells were co-treated with LPS (to induce NO production) and a test sample to determine if any samples could inhibit NO production consistent with anti-inflammatory activity. Anti-inflammatory activity was compared with the action of pure quercetin, a plant-derived phytosterol and known anti-inflammatory compound. Again, to ensure that any treatment effect was not related to toxicity, cell viability was measured in the assay in response to all project samples and ranged from 70–85 percent (expressed as a percentage compared to untreated cells).
The following method was followed:
- 7 cells were cultured in flasks and maintained at 37 degrees-C with 5 percent CO2 in growth media (DMEM, 10 percent fetal bovine serum).
- Cells were cultured until approximately 90 percent confluent before being collected and counted.
- 6×1057 cells/mL were plated into clear 96-well plates and cultured for 2 days in the presence of water extracts prepared from project samples (see Table 1) and LPS diluted in growth media.
- After two days, cell culture media was transferred to black 96-well plates for nitrite production (i.e., detection of nitric oxide) using DAN (2,3-Diaminonaphtaline).
- After 10 minutes, fluorescence was measured (Ex 360 nm; Em 430 nm) and plotted against a nitrite standard curve.
- Results were further extrapolated using an LPS-nitrite production standard curve and compared with a quercetin dose response.
- The cells remaining in the clear plates were washed before cell viability was measured at 490 nm using CellTiter96 Aqueous One Solution Cell Proliferation Assay (Promega).
Statistical analyses
Results were analyzed for significant differences using one-way analysis of variance (ANOVA) followed by Tukey’s or Dunnett’s multiple comparison test. Before analyses, the ANOVA assumptions of normality of residuals and homogeneity of variances were tested using the Shapiro-Wilk and Levene tests, respectively. Differences were deemed significant when P<0.05. All statistical analyses were performed using GraphPad Prism 9 software.
Chemical composition of the test ingredients

All test aquafeed ingredients, except the commercial cricket powder, were analyzed by CSIRO staff using established best-practice methods at the Queensland Bioscience Precinct Analytical Laboratory. The cricket powder composition was provided by the supplier. Analyses were based on the Association of Official Agricultural Chemists (AOAC, 2016).
Dry matter content was determined by gravimetric analysis following drying at 105 degrees-C for 16 hours. Ash content was determined based on mass change after combustion in a muffle furnace (1521CAF Ashing Furnace, S.E.M Equipment, Australia) at 550 degrees-C for 16 hours. The total lipid portion was extracted according to the method proposed by Folch et al. (1957) and used to determine crude lipid content. Measurement of total nitrogen (N) content was undertaken using a CHNS/O organic elemental analyzer (Thermo Fisher Scientific, Waltham, Mass., USA) and used to calculate sample crude protein content based on N x 6.25. Carbohydrate content was calculated by difference. The chemical composition of the various samples is shown in Table 2.
Results and discussion
Based on the in vitro findings from this study, BSF larvae contain immunogenic molecules, with the potential to modulate immunity in vivo, and anti-inflammatory molecules that may alleviate inflammatory responses in vivo. Findings from this project show that BSF organic products may be used as a functional feed additive for aquafeeds, and that refining the chemical composition of BSF organic products could improve its value as an alternative protein source, depending on the target aquaculture species.
Immunogenic (or pro-inflammatory) capacity in vitro
Immunogenic (or pro-inflammatory) capacity (expressed as ng LPS equivalents per gram of starting material) is shown in Fig. 1 and was significantly higher in BSF Pre and Post samples compared to other test ingredients. Furthermore, BSF Post exhibited significantly higher immunogenic capacity compared to BSF Pre.
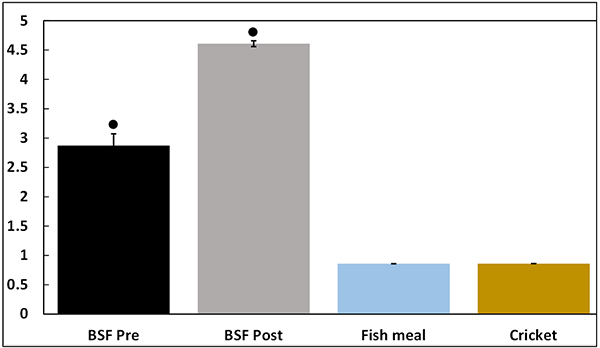
*Denotes significant differences compared to cricket powder using a one-way analysis of variance followed by Tukey’s multiple comparison test.
Recent in vivo studies have explored the immunogenic capacity of supplementing animal feed with BSF larvae (references available from the corresponding author) and observed positive impacts on growth, gut health and immune response. Recent in vitro studies have also explored the immunogenic capacity of BSF larvae. For example, Ali and co-workers explored NO production by RAW264.7 cells in response to BSF larvae extracts and a novel bioactive polysaccharide (named dipterose-BSF) purified from BSF larvae extracts. Dipterose-BSF even stimulated NO production similarly to LPS. Thus, these published findings support the outcomes from the present study and suggest that meals or extracts prepared from BSF larvae may improve animal health through immunomodulation when included in the diet.
In the present study, BSF Pre and BSF Post exhibited higher immunogenic activity than cricket powder and fishmeal. Furthermore, BSF Post significantly increased NO production more than BSF Pre suggesting that expeller pressing may improve the immunogenic capacity of BSF. This may be due to the enrichment of immunogenic molecules or improved solubility following expeller pressing. Additional processing may further improve the immunogenic capacity of BSF Post. Although one could speculate that the pro-inflammatory property of BSF may make high inclusion rates less desirable, particularly if the immune system is overly stimulated, although current literature suggests no impairments in this context (as described below).
Dietary evaluation of black soldier fly larvae meal on growth and health of Pacific white shrimp
Anti-inflammatory capacity in vitro
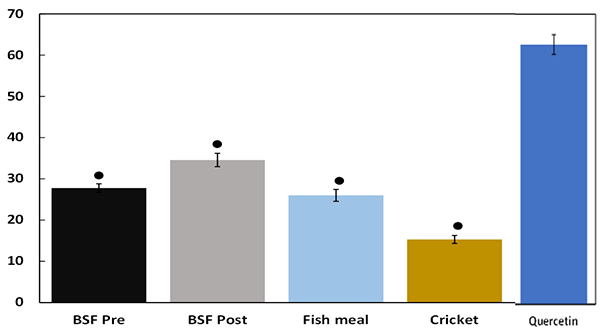
Anti-inflammatory capacity (expressed as percentage inhibition of NO production) is shown in Fig. 2 and was similar in response to BSF Pre, BSF Post, and fishmeal samples. Compared to cell treatment with quercetin (a plant-derived anti-inflammatory molecule), anti-inflammatory capacity was significantly lower in response to BSF Pre, BSF Post, fishmeal and cricket powder. However, BSF Pre and BSF Post samples exhibited significantly higher anti-inflammatory activity compared to cricket powder.
A recent in vivo study explored the impact of supplementing aquafeeds with BSF in rainbow trout revealing evidence of decreased intestinal inflammation, particularly when supplemented with soy-based diets known to induce inflammation and reduce the integrity of the intestinal barrier. In the current study, anti-inflammatory capacity was similar in response to BSF Pre, BSF Post and fishmeal samples and significantly lower than the cellular response to quercetin. However, BSF Pre and BSF Post samples exhibited significantly higher anti-inflammatory activity compared to cricket powder.
Chemical composition
The chemical composition of the BSF organic meals makes them desirable ingredients for aquafeeds and is within the range reported in the literature. Those products are rich in protein and contain relatively low levels of ash and carbohydrates compared to other alternative protein sources. Reducing the lipid content of BSF meals should be considered to help achieve better nutritional value for the aquafeed market (e.g., lipid levels between 5–12 percent depending on the targeted aquaculture species). Lower lipid levels in BSF meals will consequently increase protein and carbohydrate content and likely increase the immunogenic and anti-inflammatory capacity, as discussed below.
Rombenso, BSF, Table 2
| Composition measure | BSF Pre | BSF Post | Fishmeal | Commercial cricket powder* |
|---|
Composition measure | BSF Pre | BSF Post | Fishmeal | Commercial cricket powder* |
|---|---|---|---|---|
| Dry matter (%) | 86.4 | 87.9 | 87.3 | – |
| Ash (%) | 9.2 | 10.2 | 15.3 | – |
| Total lipid (%) | 21 | 14.7 | 10.6 | 9.5 |
| Crude protein (%) | 45.8 | 50.4 | 72.9 | 68.5 |
| Carbohydrate (%) | 10.2 | 12.5 | 1.2 | 1 |
* Sample composition supplied with sample.
Final considerations
BSF organic insect meal contains immunogenic molecules that were pro-inflammatory and induced NO production significantly higher than fishmeal and cricket powder. Notably, expeller pressing significantly improved the immunogenic capacity of BSF organic insect meal by 60 percent. BSF organic meals appear to be suitable as ingredients and may have functional properties of strategic relevance to aquafeeds. Optimizing the composition of the meals, particularly by defatting to increase protein content, is recommended to refine this product for the nutritional needs of carnivorous aquaculture species.
Further in vitro studies could explore different processing techniques and examine the different macronutrient fractions that best enhance the immunogenic potential of BSF organic insect meal. Animal studies could also be considered to investigate the potential of BSF organic insect meal at different inclusion rates (functional additive <10 percent; protein source 10–40 percent) to improve gut health, reduce the severity of bacterial/viral diseases, and be used as a commercially cost-effective alternative protein source.
References are available from the corresponding author.
Now that you've reached the end of the article ...
… please consider supporting GSA’s mission to advance responsible seafood practices through education, advocacy and third-party assurances. The Advocate aims to document the evolution of responsible seafood practices and share the expansive knowledge of our vast network of contributors.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year.
Not a GSA member? Join us.
Authors
-

Artur Rombenso, Ph.D.
Corresponding author
Senior Research Scientist – Animal Nutrition
CSIRO Livestock & Aquaculture Program
Bribie Island Research Centre, 144 North St, Woorim, QLD 4507 AUS -
Anca Rusu
Research Technician – Molecular Nutrition
CSIRO Livestock & Aquaculture Program
Queensland Bioscience Precinct, 306 Carmody Road, St Lucia, QLD 4067 AUS -
Andrew Porter
CEO
BSF Organic Pty. Ltd
1485 Ballan Road, Anakie, VIC, 3213 -
Nicholas Bourne
Research Technician – Animal Nutrition
CSIRO Livestock & Aquaculture Program
Queensland Bioscience Precinct, 306 Carmody Road, St Lucia, QLD 4067 AUS -
Ha Truong, Ph.D.
Research Scientist – Animal Nutrition
CSIRO Livestock & Aquaculture Program
Bribie Island Research Centre, 144 North St, Woorim, QLD 4507 AUS -
Cedric Simon, Ph.D.
Principal Research Scientist – Animal Nutrition
CSIRO Livestock & Aquaculture Program
Queensland Bioscience Precinct, 306 Carmody Road, St Lucia, QLD 4067 AUS -
Simone Osborne, Ph.D.
Senior Research Scientist – Molecular Nutrition
CSIRO Livestock & Aquaculture Program
Queensland Bioscience Precinct, 306 Carmody Road, St. Lucia, QLD 4067 AUS
Tagged With
Related Posts

Aquafeeds
Black soldier fly larval production in a stacked production system
Study describes development and evaluation of an “all-in-one” stacked system for indoor production of black soldier fly larvae.

Aquafeeds
Meta-analysis of the effects of black soldier fly meal on fish growth
Results indicate it is feasible to achieve high levels of substitution of fishmeal with black soldier fly meal without risking a negative impact.

Aquafeeds
Improving the lipid profile of black soldier fly larvae
Black soldier fly larvae fed with marine-based substrates displayed an improved lipid profile with higher polyunsaturated fatty acid levels.

Aquafeeds
Fly guys: Canada opens the door for insect-based feed companies
The sci-fi flick “The Fly” warned about mixing flies and technology, but high-tech black soldier fly farmers are seizing a real opportunity in aquaculture.



