NOAA, UNC-Wilmington collaboration identifies swim bladder overinflation, other challenges
 The red porgy (Pagrus pagrus), also known as sea bream and silver or pink snapper, is a highly valued marine finfish in the family Sparidae. Red porgy are found in the Mediterranean Sea, eastern Atlantic from the British Isles to Senegal, and western Atlantic from North Carolina, USA, to Mexico, and Venezuela to Argentina. In the coastal Atlantic Ocean off the southeastern United States, red porgy are an important member of the snapper-grouper complex, with stocks currently classified as overfished.
The red porgy (Pagrus pagrus), also known as sea bream and silver or pink snapper, is a highly valued marine finfish in the family Sparidae. Red porgy are found in the Mediterranean Sea, eastern Atlantic from the British Isles to Senegal, and western Atlantic from North Carolina, USA, to Mexico, and Venezuela to Argentina. In the coastal Atlantic Ocean off the southeastern United States, red porgy are an important member of the snapper-grouper complex, with stocks currently classified as overfished.
Red porgy are protogynous hermaphrodites, beginning life as females and then transforming into males. The seasonal change in photoperiod between winter and spring appears to stimulate gonadal maturation, with spawning typically occurring in late winter to early spring.
Commercial production of the red porgy has been demonstrated in the Mediterranean and South America. Due to the high market value of red porgy in U.S. markets and the overfished status of red porgy populations, the fish appears to be a viable candidate for culture in North America.
Collaborative project
In January 2005, a collaborative partnership among the National Oceanic Atmospheric Administration (NOAA) National Ocean Service, National Marine Fisheries Service and University of North Carolina Wilmington Marine Science Aquaculture Program was developed to study aquaculture of the red porgy.
The primary objective of this project was to develop methods for broodstock conditioning, egg production, larval culture and juvenile growout. Secondly, it compared the culture performance of Atlantic red porgy with the Mediterranean red porgy to investigate the potential for red porgy culture in North America.
The research was funded by NOAA, the North Carolina Fishery Resource Grant Program and United States Department of Agriculture Cooperative State Research, Education and Extension Service.
Broodstock conditioning
In March 2004, red porgy broodstock were collected off the coast of North Carolina, USA, at a depth of approximately 30 m using rod and reel. The fish were transported to the aquaculture facilities of the NOAA Center for Coastal Fisheries and Habitat Research in Beaufort, North Carolina.
All fish collected experienced overinflation of the swim bladder and required ventilation by needle at the time of capture. All returned to normal orientation within 24 hours. After an 18-day acclimation period, 20 fish averaging 30.6 cm and 373 grams were selected as broodstock and moved to a 30-cubic-meter outdoor tank. Water temperature was adjusted monthly to reflect North Carolina offshore bottom temperatures at depths of 33-37 meters.
Approximately 15 to 20 percent new seawater was exchanged weekly using ambient water from the estuary. Temperatures in the broodstock tank were 30 degrees-C during the late summer months of July and August and 15 degrees C during the winter months of January and February. Under these conditions, the 20 red porgy broodstock produced up to 300,000 viable eggs daily during their natural spawning period between January and March 2005, with peak spawning in mid-February (Fig. 1).

Larval culture

For larval culture experiments, eggs were transported in a temperature-controlled plastic bag with seawater and supplemental oxygen to the University of North Carolina Wilmington Aquaculture Facility in Wrightsville Beach, North Carolina. Between January 28 and March 3, the survival and growth of larval red porgy from egg through the metamorphic stages were studied under pilot-scale hatchery conditions.
The larval-rearing system consisted of three black 155-l cylindrical tanks, each supplied with 1-µ-filtered flow-through full-strength seawater at an exchange rate of 2.5 times per day. Constant light intensity was maintained at the water surface with 18 hours of daily light. The temperature was kept at 22 degrees-C.
Nannochloropsis oculata algae was added twice daily to maintain a density of 300,000 cells/ml from one day post-hatch until the end of the rotifer feeding period. Three days post-hatch, enriched Brachionus rotundiformis rotifers were added to each rearing tank at a density of 6 rotifers/ml. Once feeding commenced, rotifer density was increased to 20/ml.
The larvae also received artificial microdiets. Newly hatched artemia nauplii were introduced at a rate of 0.25/ml 12 days post-hatch, and by 17 days, larvae were fed enriched artemia at a density of 1.2/ml. By day 26, the larvae were completely weaned to artificial dry diets.
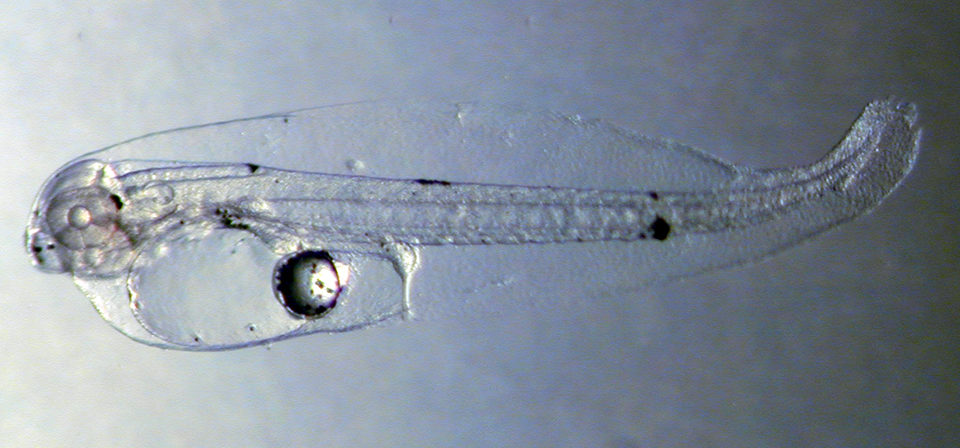
Larval survival to day 10 post-hatch was 75.0 ± 2.2 percent. At this time, swim bladder overinflation contributed to a significant decline in survival that reached 2.4 percent by day 35, producing 1,200 post-metamorphic stage juveniles. Larvae reached 11.20 ± 1.12 mm and 29.30 ± 0.55 mg on day 35, and juveniles reached a 55-mm total length 90 days post-hatch.
Juvenile grow-out
After 36 days, juveniles were transferred from larval-rearing tanks to three 160-liter flow-through raceways. Fish were fed a commercially prepared diet containing 55 percent protein and 15 percent fat. Environmental conditions were 12 hours light and 22 degrees-C.
At 55 days post-hatch, fish averaged a 0.58-gram weight and 28.2-mm total length. They grew to 2.44 grams and 48.6 mm 20 days later. By 91 days, juvenile red porgy averaged 3.79 grams and 56.5 mm in length for an average daily weight gain of 0.08 grams and a specific growth rate of 8.7 percent/day.
At that time, 1,200 juveniles were transferred to an outdoor 11.3-cubic-meter fiberglass recirculating tank with a flow rate of 150 liters per minute. Fish were reared under ambient photoperiod at 21 degrees-C and a salinity of 34 grams per liter through December 2005. The trial resulted in red porgy reaching 195.00 ± 0.32 mm total length and 158.00 ± 0.14 grams at 313 days post-hatch. The fish had a specific growth rate of 6.8 percent per day and overall feed conversion of 1:1.
Survival remained at 94.2 percent until 210 days, when nitrogen saturation and high levels of ammonia were encountered due to mechanical problems with the recirculating system. By 224 days, survival decreased to 79.0 percent. To correct these problems, fish were temporarily transferred out of the system while repairs were performed. Survival following this transfer decreased to 65.3 percent, and final survival of the juveniles from 90 to 313 days was 32.0 percent.
Perspectives
The work with red porgy suggested a slower larval growth rate for western Atlantic red porgy compared to Mediterranean red porgy. However, juvenile growth rates were significantly higher than previously reported. Survival in later larval stages was lower when compared to the Mediterranean fish.
The authors believe larval survival can be significantly improved once the problem of swim bladder overinflation between 10 and 35 days posthatch is resolved. The cause of the bladder problem is unknown, but might be due to nutritional deficiencies, disease or tank-induced reasons such as inadequate circulation, which causes the larvae to congregate at the tank surface.
(Editor’s Note: This article was originally published in the January/February 2009 print edition of the Global Aquaculture Advocate.)
Now that you've reached the end of the article ...
… please consider supporting GSA’s mission to advance responsible seafood practices through education, advocacy and third-party assurances. The Advocate aims to document the evolution of responsible seafood practices and share the expansive knowledge of our vast network of contributors.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year.
Not a GSA member? Join us.
Authors
-
Troy C. Rezek, M.S.
University of North Carolina Wilmington
Center for Marine Science
601 South College Road
Wilmington, North Carolina 28403-5927 USA -
Wade O. Watanabe, Ph.D.
University of North Carolina Wilmington
Center for Marine Science
601 South College Road
Wilmington, North Carolina 28403-5927 USA -
James A. Morris, Jr., M.S.
National Oceanic Atmospheric Administration
National Ocean Service
National Centers for Coastal Ocean Science
Center for Coastal Fisheries and Habitat Research
Beaufort, North Carolina, USA -
Neil A. McNeill, B.S.
National Marine Fisheries Service
Southeast Fisheries Science Center
Beaufort, North Carolina, USA
Related Posts

Intelligence
A brief look at genetically modified salmon
If approved by FDA, fast-growing genetically modified salmon will provide a safe and nutritious product similar to other farmed Atlantic salmon.

Intelligence
A land grab for salmon (and shrimp) in upstate New York
The operators of Hudson Valley Fish Farm see their inland locale as a pilot to prove that land-based fish farming, located in close proximity to major metropolitan markets, can be successful.
Aquafeeds
Alternative feed ingredients support continued aquaculture expansion
Identifying sources for essential macro- and micronutrients is important, as well as understanding how best to manufacture feed to required physical specifications when using these new raw materials.
Health & Welfare
Animal health giants have sea lice in their crosshairs
Alltech and Benchmark have been working on the next generation of sea lice solutions and believe they have new products that can help salmon farmers win.


