
Intensification of aquaculture systems makes fish more susceptible to stress and disease. Aquafarmers currently treat with drugs such as antibiotics or use vaccines as a means of controlling disease. Another approach is to cull diseased fish and replace them with new stocks after disinfecting the system. However, there are problems associated with these methods.
Antibiotic-resistant strains of bacteria are increasing, and some antibiotics have been shown to be immunosuppressive. Commercially available vaccines have proven very successful in reducing disease outbreaks, but there is still an insufficient number available to cover all the diseases that currently affect the industry.
Genetic selection and disease resistance
Genetic selection as a means of improving disease resistance of stocks is presently receiving much attention. Some strains of fish are naturally resistant to a particular pathogen, and are very useful for studying the underlying mechanisms involved in disease resistance.
Inbred strains
Inbred strains of fish produced in the laboratory using conventional sib-mating are ideal for immunological studies. Phenotypes for different immune traits can be defined in the different strains, and these in turn can be related to disease resistance. However, these strains can take 15-20 generations to develop.
Inbred homozygous clones
Gynogenetic reproduction offers a rapid alternative for developing fully inbred homozygous clones (IC) of fish within two generations. Since the clones have identical genotypes, the genetic variability between clones can be monitored, and clones with favorable or unfavorable genes for disease resistance can be identified.
Immune mechanisms
Although most fish are susceptible to initial infection by an invading pathogen, they appear to differ in their ability to limit the infection through a variety of immune mechanisms. Once a pathogen has breached the physical barriers of its host, it encounters components of the nonspecific immune response.
These include serum lysozyme, the complement cascade, lectins, and C-reactive protein, which attack and help destroy the pathogen. These molecules also coat the pathogen for further destruction by phagocytes, cellular components of the nonspecific immune system, which engulf and destroy invading pathogens.
Clonal line study
A study at the University of Stirling’s Institute of Aquaculture in Scotland examined the genetic variation of clonal lines of Nile tilapia (Oreochromis niloticus L.) in relation to disease resistance. Mitotic gynogenesis was used to produce a first generation of completely homozygous female Nile tilapia. Three inbred clones (IC A, IC B, and IC C) were then established from mitotic gynogenetic females by meiotic gynogenesis. Three gynogenetic outbred clones (OC) – OC AxB, OC BxC, and OC CxA – were also produced by crossing the inbred clones. A group produced by ordinary crossing was used as an unrelated control.
Nonspecific immune responses
Nonspecific immune responses (serum lysozyme activity, respiratory burst, and phagocytosis of bacteria by head kidney macrophages) were compared between the clones. Differences were found in the number of macrophages containing phagocytosed bacteria. A significantly higher percentage of macrophages containing phagocytosed bacteria were seen in IC A, IC C, and OC AxB compared with IC B. The remaining groups had values intermediate to these.
The percentage of macrophages containing phagocytosed bacteria in OC AxB fish was not significantly different than that of IC A, but was significantly higher than in IC B fish. The results suggested that the inheritance of this immune activity shows dominance toward the stronger phagocytic activity.
The serum lysozyme activity of IC C was significantly higher (p<0.05) than in IC A and IC B. Significantly higher (p<0.05) lysozyme activity was present in OC BxC and OC CxA fish compared with IC A, IC B, and OC AxB fish, but lysozyme activity was significantly lower than in IC C fish. The OCs showed an intermediate level of lysozyme activity to that of their parents. Their intermediate response suggested additive parental genetic effects in the outbred offspring.
Establishing genetic differences
Ultimately, the best way to establish genetic differences for disease resistance between the clones was to determine their survival rate after artificially infecting them with a pathogen. Fish injected with Aeromonas hydrophila started to die within 12 hours after injection. A. hydrophila infection was established from kidney swabs taken from dead animals. All dead fish showed typical symptoms of A. hydrophila infection, including hemorrhaging on the body surface, base of fins, and operculum (Fig. 1).
Cumulative mortalities in IC B reached 56.7 percent over the six-day experimental period (Table 1). Mortalities were also observed in the other lines, but at a much lower level. Only 3.3 percent of IC A and 6.7 percent of IC C died over the experimental period, with both showing a strong resistance to the pathogen. The outbred clones OC AxB, OC BxC, and OC CxA also showed low levels of mortality (20 percent, 13.3 percent and 10 percent, respectively).
Thompson, Response of clonal lines of Nile tilapia to an artificial challenge with Aeromonas hydrophila, Table 1
| * Clone | Infected Fish Mortalities | Infected Fish Survivors | Noninfected Fish |
|---|---|---|---|
| IC Aa | 1 | 0 | 29 |
| IC Bc | 17 | 5 | 8 |
| IC Cab | 2 | 0 | 28 |
| OC AxBb | 6 | 0 | 24 |
| OC AxCab | 4 | 1 | 25 |
| OC CxAab | 3 | 2 | 25 |
Table 1: Response of clonal lines of Nile tilapia to an artificial challenge with Aeromonas hydrophila. Clones with different superscript letters indicate significant differences in levels of infection at P<0.5.
Reisolating pathogen
It was also possible to re-isolate A. hydrophila from surviving fish at the end of the experiment. Only IC B were still infected with the pathogen (16.7 percent) among the IC groups, while OC BxC (3.3 percent) and OC CxA (6.7 percent) still carried the pathogen among the OC groups. The IC B fish appeared to be the most susceptible to A. hydrophila infection, with the total number of infected fish in this group (mortalities and infected survivors) significantly higher than all other fish groups.
Crossbreeding IC B with the other inbred lines, IC A and IC C, produced more disease-resistant hybrids. These outbred clones, OC AxB and OC BxC, showed significantly higher resistance to A. hydrophila than their disease-susceptible parental clone, IC B. The results of the challenge experiment suggested dominance in inheritance of susceptibility to infection in the direction of the more resistant parental clone.
Genetic variation between clones
The significant differences between the inbred clones for nonspecific immune responses and levels of infection with A. hydrophila strongly indicated the genetic variation between the clones for these parameters. Cumulative mortalities of fish in the study showed that when an IC susceptible to A. hydrophila was crossed with a resistant IC, the resulting progeny exhibited intermediate levels of resistance to that of their parents.
There was some evidence of heterosis in the form of dominance in the inheritance of some of the parameters studied, in that where inheritance appeared to be nonadditive (i.e., not intermediate between the two IC parental clones), the phenotypic values for the OC groups tended to be closer to that of the parental IC with the stronger immune response. However, none of the tilapia OC groups exceeded the highest parental value shown by the two parental lines for any of the parameters measured.
Conclusion
This university study created clones of Nile tilapia in order to evaluate variations in disease resistance due to nonspecific immunity. A positive correlation was obtained between the level of infection with A. hydrophila and the parameters measured. Additional work is now being carried out to further phenotype the clonal lines of Nile tilapia to understand the genetic control of the immune response and its role in disease resistance in these fish.
(Editor’s Note: This article was originally published in the February 2002 print edition of the Global Aquaculture Advocate.)
Now that you've reached the end of the article ...
… please consider supporting GSA’s mission to advance responsible seafood practices through education, advocacy and third-party assurances. The Advocate aims to document the evolution of responsible seafood practices and share the expansive knowledge of our vast network of contributors.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year.
Not a GSA member? Join us.
Authors
-
Kim D. Thompson, Ph.D.
Institute of Aquaculture
University of Stirling
Stirling FK9 4LA
Scotland, United Kingdom[107,117,46,99,97,46,114,105,116,115,64,49,116,100,107]
-
David J. Penman, Ph.D.
Institute of Aquaculture
University of Stirling
Stirling FK9 4LA
Scotland, United Kingdom -
Brendan J. McAndrew
Institute of Aquaculture
University of Stirling
Stirling FK9 4LA
Scotland, United Kingdom -
M. Rafiq I. Sarder, Ph.D.
Department of Fisheries Biology and Genetics
Bangladesh Agricultural University
Mymensingh, Bangladesh
Tagged With
Related Posts

Health & Welfare
Performance testing of clonal Nile tilapia lines
An approach to improve growth performance and phenotypic uniformity is the reduction of genetic variability by production of clonal Nile tilapia lines.
Responsibility
On GM foods, part 2: Let’s talk about what truly matters
With entrenched positions on GM foods, it is difficult to change the conversation, but change it we must. A perpetually contentious climate doesn’t serve us well and we must abandon the current paradigm to define a new framework that facilitates more useful discussions.
Health & Welfare
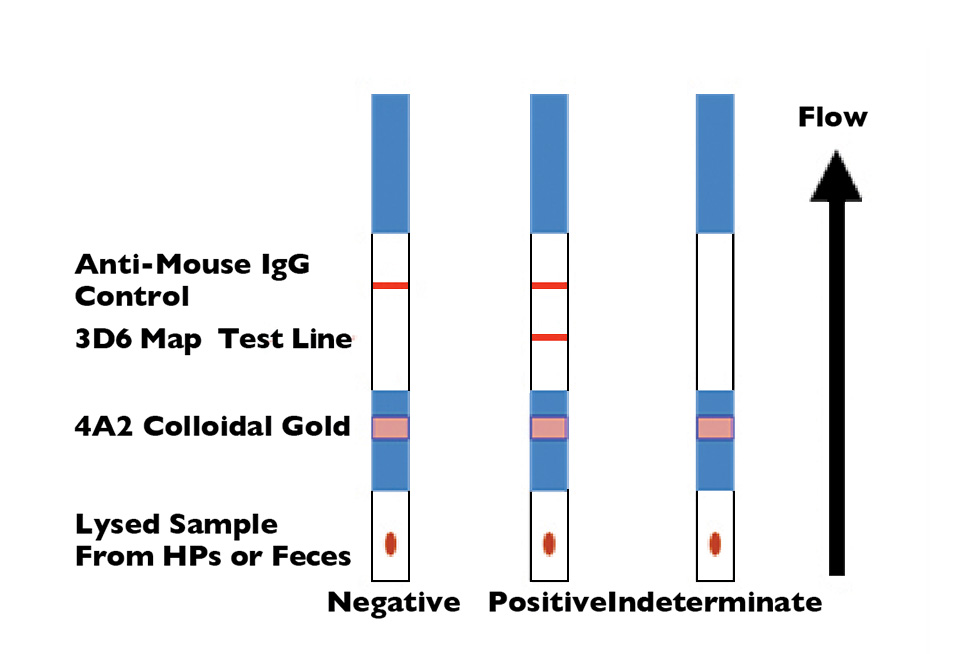
Rapid test detects NHP in penaeid shrimp
A rapid lateral-flow immunoassay using monoclonal antibody combinations can identify necrotizing hepatopancreatitis (NHP) infection in less than 15 minutes.
Health & Welfare
Diagnostic profile of Belize Taura Syndrome Virus
Shrimp farming in Belize, Central America, was hard hit by Taura Syndrome Virus (TSV, now known as TSV serotype A) in 1996 and a re-emergence in 2002.


