Treatment with coagulants, particularly alum, almost always clears turbidity from ponds

Excessive turbidity in ponds caused by suspended soil particles is a frequent occurrence – especially in freshwater aquaculture. The most effective means of preventing “muddy” ponds is to control erosion both in pond watersheds and within ponds.
Where turbid water supplies cannot be avoided, sedimentation reservoirs allow water to be cleared of excessive turbidity before it is transferred to ponds. In some cases, however, ponds may remain turbid after all usual measures of controlling erosion and removing turbidity from inflow have been implemented.
Particle settling
Soil particles settle from water in response to gravity, and larger particles settle faster than smaller particles of equal density. The density of mineral soil is more than twice that of organic matter. Thus, mineral matter settles much faster than organic matter of the same particle size.
Troublesome turbidity usually results from clay or fine silt particles. These particles remain in suspension, causing prolonged turbidity for three basic reasons. A water body may have enough turbulence to prevent fine particles from settling. The resuspension rate of particles may exceed the rate of sedimentation. In addition, colloidal clay settles at an exceedingly slow rate.
Clay particles are negatively charged, and mutual repulsion counteracts the tendency of particles to floc together and settle. The presence of cations tends to neutralize negative charges on clay particles and allow them to floc. The capacity of cations to floc clay particles increases with increasing valence.
Monovalent cations (sodium and potassium) are much less effective as flocculants than divalent cations (calcium and magnesium). Trivalent cations such as aluminum and ferric iron are particularly strong flocculants, but their natural concentrations in water are very low.
The rate that colloidal clay particles settle from water becomes greater as the total dissolved solids concentration (conductivity or salinity) rises. This tendency is much stronger if water hardness (greater calcium and magnesium concentration) also increases at higher total dissolved solids concentrations.
Biological processes also can cause clay particles to settle. Bacteria, phytoplankton and decaying particles of organic matter have mucilaginous surfaces to which clay particles stick, allowing flocculation to begin. Also, changes in water pH resulting from photosynthetic activity or decomposition of organic matter sometimes alter conditions on or around clay particles and allow them to flocculate and settle.
Alkalinity
When waters do not clear after sources of turbidity have been eliminated, the total alkalinity should be tested. If alkalinity is low – less than 30 mg/L – agricultural limestone should be applied at 2,000 to 3,000 kg/ha. Alternatively, lime can be applied, but if the pond has been stocked, lime should not be applied at more than 50 kg/ha to avoid high pH. Repeated doses of lime can be made at weekly intervals.
In ponds that have alkalinity above 40 mg/L, or if liming does not clear turbidity, there are two options: Attempt to clear turbidity using fertilizers or treat ponds with coagulants. The common approach is to try fertilizers and use coagulants as the last resort.
Aquaculture ponds, coagulants
Most aquaculture ponds with excessive turbidity receive manures, chemical fertilizers or feeds. In manured or fed ponds, one or more applications of urea and triple superphosphate or other nitrogen and phosphorus fertilizers can cause clay particles to settle.
In turbid, fertilized ponds, application of animal manure, grass or other organic material may clear turbidity. Large inputs – 1,000-2,000 kg/ha – of organic matter are needed, and it often is necessary to apply the organic matter in several small applications a few days apart to avoid dissolved oxygen depletion.
The many possible coagulants include calcium sulfate (gypsum), calcium chloride, aluminum sulfate (alum), aluminum chloride, iron sulfate, iron chloride and certain organic polymers. The two most common coagulants used in aquaculture ponds are gypsum and alum.
Gypsum
Gypsum must be applied in large amounts to remove turbidity. The usual treatment rate is 100-150 mg/L, but tests may be made in jars to ascertain the lowest effective treatment rate. In a 1-ha, 1-m-deep pond, 100 mg/L would require a dose of 1,000 kg/ha.
Gypsum should be spread over the pond surface, and as it dissolves, it will increase the calcium concentration in the water to improve conditions for flocculation of suspended particles. The effective treatment rate for calcium chloride is similar to that of gypsum.
Alum
Doses of iron and aluminum compounds needed to clear turbidity are lower than those needed for calcium compounds. For example, filter alum usually is effective at concentrations of 20-50 mg/L, and jar tests can ascertain optimum application rates. Alum is potentially dangerous because it is highly acidic, and workers who handle it should wear protective clothing. Alum also depresses total alkalinity concentrations by roughly 0.5 mg/L for each 1.0 mg/L of this coagulant.
In most ponds, alum depresses alkalinity and pH, but after a few days, values return to initial levels (Fig. 1). However, alum treatments should not be more than 50 percent of the total alkalinity concentration, and low-alkalinity water should be limed before applying alum.
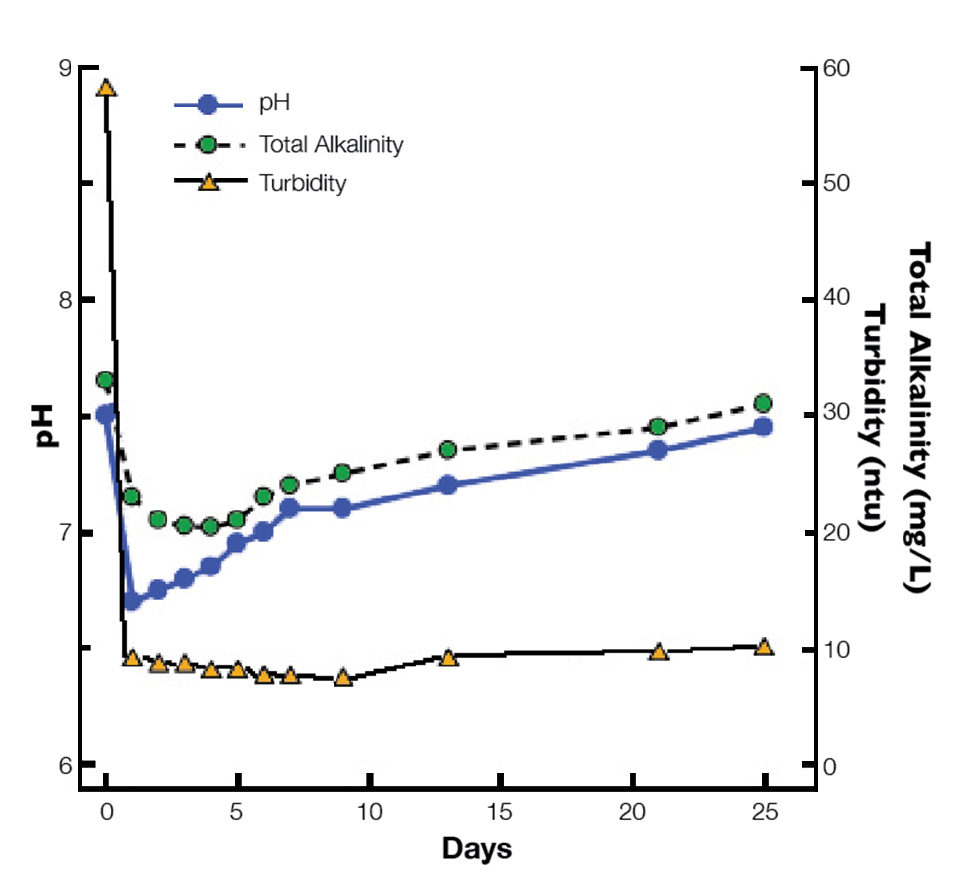
Alum should be pre-dissolved in water and applied over the entire pond water surface on a still day without rainfall. Aerators can be used for a few minutes to mix the alum with pond water, but they should then be turned off to allow the floc to settle.
It is important to apply enough alum initially to cause flocculation, because alum has no residual effect. The active ingredient, aluminum ion, precipitates from water as aluminum hydroxide within a few minutes. If the initial treatment is not sufficient, it is lost. Thus, it is prudent to make a jar test to determine the minimum effective treatment rate.
To make the test, make a 10,000 mg/L alum stock solution by dissolving 1 g alum in 100 mL water and fill 10 clear 1-L jars or beakers with the water to be tested. Make a series of alum concentrations ranging 5-50 mg/L at 5-mg/L concentration intervals by adding stock solution (0.5 mL stock solution = 5 mg/L alum) to the jars. Stir vigorously, wait one hour and determine the lowest concentration that caused turbidity to precipitate.
Eliminate turbidity sources first
Treatment with coagulants, particularly alum, almost always clears turbidity from ponds. However, turbidity may return because sources of turbidity have not been controlled, or the culture species caused bioturbation. Aerator-generated currents cause turbidity, and normal wave and wind action are sufficient to continually re-suspend particles and cause turbidity.
The best approach always is to eliminate sources of turbidity, and use alum only if turbidity remains.
(Editor’s Note: This article was originally published in the September/October 2012 print edition of the Global Aquaculture Advocate.)
Author
-

Claude E. Boyd, Ph.D.
Department of Fisheries
and Allied Aquacultures
Auburn University
Auburn, Alabama 36849 USA
Tagged With
Related Posts

Health & Welfare
Aquamimicry: A revolutionary concept for shrimp farming
Aquamimicry simulates natural, estuarine production conditions by creating zooplankton blooms as supplemental nutrition to the cultured shrimp, and beneficial bacteria to maintain water quality. Better-quality shrimp can be produced at lower cost and in a more sustainable manner.

Health & Welfare
Biofloc technology holds potential for carnivorous fish species
Juvenile carnivorous African catfish performed well in biofloc-based systems, which could help produce better quality and more disease-resistant seed of this important aquaculture species and support the expansion of African catfish farming industry.

Responsibility
Calcium and magnesium use in aquaculture
Aquatic plants and animals get the essential nutrients calcium and magnesium from water and food. Calcium concentrations impact the hydration and development of eggs in a hatchery, where calcium carbonate precipitation can be troublesome.

Uncategorized
Change the paradigm and break aquaculture stereotypes
Although there is compelling evidence that early humans relied heavily on the fruits of the ocean, the mindset of powers that be tends to focus on utilization of land for food. In Australia, for example, the country controls water resources with 2.5 times the area of its land, yet it is not food secure in seafood. Many bright minds have not awakened to the unique opportunities that aquaculture and fisheries offer. It’s time to get aquaculture out of the pigeonhole.

