Aquaculture Pathology Laboratory finds conclusive evidence that EHP is not the only pathogen associated with the presence of White Feces Syndrome in Litopenaeus vannamei
The shrimp gut microbiota is fundamental to the host’s nutrition, growth, pathogen resistance and maintenance of the internal steady state or homeostasis. Feeding characteristics of shrimp, such as actively grazing and cannibalism, make the host vulnerable to pathogen invasion. Colonization by alternative microorganisms destabilize the intestinal microbiota, which leads to infections and co-infections in a taxonomically diverse gastrointestinal (GI) tract.
Although there is limited information on specific immune mechanisms provided by the shrimp gut microbiota, there is evidence of its interplay with the digestive activities that directly affect shrimp growth and severity of diseases. Likewise, new emerging diseases were found to target the shrimp hepatopancreas [digestive gland] and causing growth inhibition, size disparity, loss of appetite and chronic mortality. Recently identified shrimp diseases that exhibit these clinical signs are the hepatopancreatic microsporidiosis (HPM) caused by Enterocytozoon hepatopenaei (EHP) and White Feces Syndrome (WFS), whose causative agent or agents remain unidentified.
HPM is a disease of the hepatopancreas in penaeid shrimp caused by several species of microsporidians [spore-forming, unicellular parasites] including EHP. This microsporidian species has been found in several Asian countries such as China, Vietnam, Malaysia, Indonesia, Thailand, and India. Recently, EHP has been reported in the Western Hemisphere in South America. The main clinical sign of EHP at the farm level is shrimp growth retardation, leading to an increased size variability and feed conversion rate (FCR). In advanced stages of the disease, EHP-infected shrimp typically display soft shells and chronic mortalities. EHP has been reported in several species of farmed penaeid shrimp, including black tiger prawn P. monodon, L. vannamei and P. stylirostris. EHP is an intracellular parasite that proliferates within the cytoplasm of the affected tubule epithelial cells in the hepatopancreas.
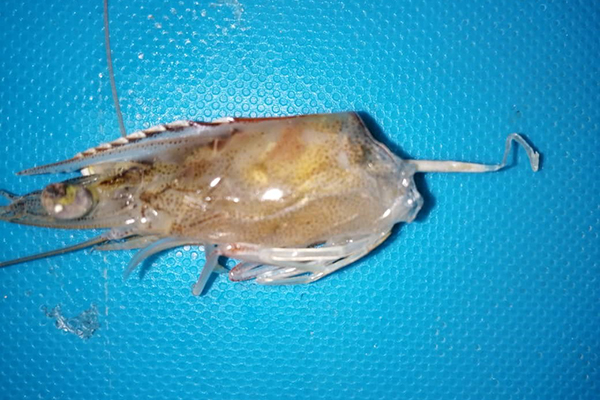
At the farm level, WFS manifests as white fecal strings floating along the surface water in grow-out ponds. Shrimp in these ponds display unusual clinical signs such as poor growth rate, size disparity, gastrointestinal tract with yellowish to whitish discoloration, softshell, and chronic mortalities. Although an association between EHP and WFS has long been suggested, no studies have successfully reproduced this syndrome in a controlled laboratory setting. Even when severe EHP infections occur, WFS does not always manifest, and these observations suggests that WFS is the result of an interplay of more than one microorganism and another causative agent(s) are yet to be identified. A strong association between WFS and EHP was reported in EHP-endemic regions in Southeast Asia and Venezuela. In these cases, WFS and EHP were detected along another opportunistic pathogen, Vibrio spp., that causes Septic Hepatopancreatic Necrosis (SHPN).
This article – adapted and summarized from the original publication (Aranguren, L.F. et al. 2021. Experimental reproduction of White Feces Syndrome in whiteleg shrimp, Penaeus vannamei. PLoS ONE 16(12): e0261289) – reports on our study to isolate and identify a strain of Vibrio parahaemolyticus as the missing link between EHP and WFS. Co-infecting EHP-infected shrimp by an immersion challenge with a unique strain of V. parahaemolyticus that was isolated from gut of a shrimp (L. vannamei) displaying WFS allowed us to provide the first experimental evidence of WFS reproduction. To our knowledge, this is the first demonstration of the reproduction of WFS under controlled laboratory conditions using two unique pathogens.
Study setup
Specific pathogen free (SPF) L. vannamei were obtained from the Oceanic Institute (Oahu, Hawaii). For at least two consecutive years, this population has tested negative by PCR for all OIE-listed pathogens as well as OIE non-listed pathogens, including EHP. The bioassay was carried out in the Aquaculture Pathology Laboratory of the University of Arizona. All procedures for the sampling and euthanasia of shrimp were performed following the guidelines established by the American Veterinary Medical Association.
For detailed information on the experimental design and animal husbandry; isolation of Vibrio parahaemolyticus from L. vannamei shrimp displaying WFS and inoculum preparation; experimental challenge to reproduce WFS; histopathology and in-situ hybridization; PCR and quantitative PCR for detection of V. parahaemolyticus and quantification of EHP; and statistical analyses, refer to the original publication.
Results and discussion
Results of our study describe for the first time the experimental reproduction of WFS in shrimp, L. vannamei pre-infected with EHP (primary pathogen) and challenged with a particular V. parahaemolyticus (secondary pathogen) isolated from the GI tract of a shrimp displaying WFS. Our data provides empirical support of a strong association of EHP with WFS as recorded in different regions of the world. In Indonesia, from 2016 to 2018, and in Venezuela in 2019, we observed shrimp with clinical signs of WFS displayed histological lesions typical of EHP and SHPN. Our histological findings revealed a similar scenario, as shrimp experiencing clinical manifestations of WFS under laboratory-challenged conditions also displayed histological lesions that are reminiscent of EHP and SHPN pathology.
Aranguren, WFS, Table 1
| Trial | Tank | Group | Initial number of animals | Final number of animals | Final survival (%) | CV% | WFS |
|---|
Trial | Tank | Group | Initial number of animals | Final number of animals | Final survival (%) | CV% | WFS |
|---|---|---|---|---|---|---|---|
| 1 | 1 | SPF negative control | 7 | 6 | 85.7 | 21.4 | No |
| 1 | 2 | SPF + V. parahaemolyticus | 7 | 6 | 85.7 | 27.6 | No |
| 1 | 3 | EHP positive control | 8 | 8 | 100 | 41.6 | No |
| 1 | 4 | EHP + V. parahaemolyticus | 7 | 4 | 57.1 | 40 | Yes |
| 2 | 1 | SPF negative control | 10 | 9 | 90 | 12 | No |
| 2 | 2 | SPF + V. parahaemolyticus | 10 | 8 | 80 | 10.1 | No |
| 2 | 3 | EHP positive control | 9 | 4 | 44.4 | 30.8 | No |
| 2 | 4 | EHP + V. parahaemolyticus | 8 | 2 | 25 | 40.7 | Yes |

The White Feces Syndrome has proven to have one of the most elusive etiologies of any of the shrimp disease that have been characterized to date. It has been more than a decade since the first formal report of EHP-infected shrimp showing WFS was published, although anecdotal evidence suggests WFS has been observed in shrimp grow-out ponds prior to this publication. Despite the presence of WFS over a decade, the etiology [causation] of the syndrome remains inconclusive.
There are also several publications with contradictory claims with respect to the role of EHP in WFS. For example, some authors reported WFS is not caused by EHP while others found a strong evidence of a clear link between EHP and WFS. There is one report claiming Vibrio cholerae as the etiologic agent of WFS, although this finding has not been confirmed by other researchers from anywhere else despite the fact WFS is an emergent problem worldwide.
Several other studies aimed to investigate the WFS phenomenon have also found some association between WFS and different etiologies. Fecal strings containing vermiform structures called aggregated transformed microvilli (ATM) has been reported as one of the causative agents of WFS in Thailand. WFS appears to be correlated with coinfection with EHP and other opportunistic Vibrio spp. that cause SHPN. In India, running mortality syndrome (RMS) has been reported in shrimp displaying WFS and SHPN. Researchers studying microbiome in WFS affected shrimp reported a significant bacterial increase in Candidatus Bacilloplasma and Phascolarctobacterium and decreases in Paracoccus and Lactococcus may contribute to WFS in shrimp. Heavy gregarine [intestinal parasites] infestation, vibriosis and hemocytic enteritis, some specific bacterial taxon or “pathobiome” [groups of species associated with a host that, when present, reduce host strength under proper environmental conditions], and different environmental conditions in a grow-out pond have been proposed to be the contributing factors for causing WFS.
While studies to reproduce WFS in a laboratory setting have been attempted by several authors, few have been successful in consistently reproducing this syndrome and determining the etiology. Huang et al. recently found that transplantation of the intestinal microbiota of shrimp displaying WFS to healthy shrimp leads to similar clinical manifestations as in WFS. They proposed WFS is caused by dysbiosis [microbial imbalance] in intestinal microbiota and this complex disease could be caused by particular pathogens. While a non-infectious etiology is worth further investigation, we cannot disregard the fact that WFS has been consistently associated with particular pathogens notably EHP. Furthermore, the constant co-occurrence of certain patterns of the bacterial communities in shrimp that present WFS lead us to hypothesize that WFS is the result of the synergetic effects of two pathogens with EHP possibly acting as a primary pathogen and an uncharacterized bacteria belonging to the family Vibrionaceae acting as a secondary pathogen.
Under laboratory challenge experiments, we were able to confirm the presence of white fecal strings only in shrimp that were pre-infected with EHP and subsequently exposed to the V. parahaemolyticus isolate. This demonstrates that, in order to induce clinical manifestations of WFS, both EHP and this particular V. parahaemolyticus isolate are required. Shrimp exposed only to EHP or V. parahaemolyticus did not develop WFS. Therefore, WFS manifestation involves a unique combination of two pathogens that had not been observed previously for any infectious disease of shrimp known to date.
Aranguren, WFS, Table 2
| Trial | Treatments | Clinical signs | Number of samples | EHP % detection by H&E histology | SHPN % detection by H&E histology | EHP detection by in situ hybridization | EHP detection by realtime-PCR |
|---|
Trial | Treatments | Clinical signs | Number of samples | EHP % detection by H&E histology | SHPN % detection by H&E histology | EHP detection by in situ hybridization | EHP detection by realtime-PCR |
|---|---|---|---|---|---|---|---|
| 1 | SPF negative control | No WFS | 6 | 0 | 0 | 0 | 0 |
| 1 | SPF + V. parahaemolyticus | No WFS | 6 | 0 | 0 | 0 | 0 |
| 1 | EHP positive control | No WFS | 8 | 100 | 37.5 | 100 | 100 |
| 1 | EHP + V. parahaemolyticus | WFS | 6 | 100 | 83.3 | 100 | 100 |
| 2 | SPF negative control | No WFS | 6 | 0 | 0 | NT | 0 |
| 2 | SPF + V. parahaemolyticus | No WFS | 6 | 0 | 0 | NT | 0 |
| 2 | EHP positive control | No WFS | 5 | 100 | 60 | NT | 100 |
| 2 | EHP + V. parahaemolyticus | WFS | 5 | 100 | 60 | NT | 100 |
Typically, in a shrimp grow-out pond, the cultured population starts showing the presence of EHP first. Later on, during the grow-out cycle, shrimp start developing white discoloration of the GI tract, and floating white fecal strings start appearing in the pond. By H&E [one of the main stains to study tissues] histology, we observed a similar sequential order in cellular pathology during the infection process. A primary pathogen, in this case EHP, caused the typical histological lesions and later on affected shrimp display SHPN caused by opportunistic V. parahaemolyticus that was used to challenge the EHP-infected shrimp. The histopathology image in Fig. 3D clearly displays co-infection of EHP as represented by a typical plasmodium inclusion and bacteria in the same HP tubule. This establishes the role of a primary pathogen like EHP to accentuate the impact of opportunistic bacteria like Vibrio sp. resulting WFS.
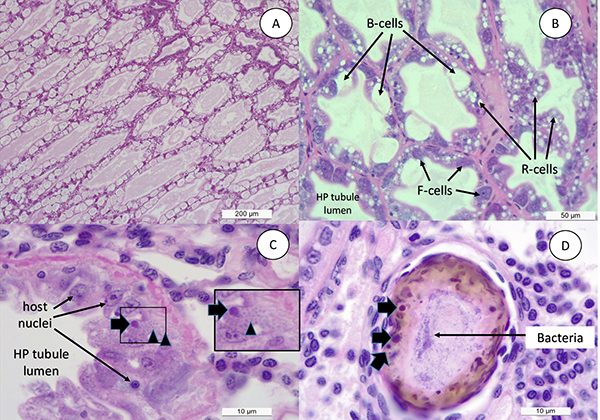
Shrimp in the treatment group EHP+V. parahaemolyticus in both of our trials displayed a high size disparity, which is higher or comparable to shrimp infected with EHP or V. parahaemolyticus alone (Table 1). A similar finding was also reported by Liu et al. (2015. Development of real-time PCR assay for detection of microsporidian Enterocytozoon hepatopenaei and detection in shrimp samples under different. Prog Fish Sci. 2015.). These authors reported the impact of EHP on size variation of shrimp in a grow-out farm when the pathogen load was found to be >103 copies/ng total DNA in hepatopancreatic tissue. In our study, the mean EHP load in shrimp displaying clinical signs of WFS was 1.4 × 107 copies/ng of extracted DNA, which is higher from what Liu et al. (2015) mentioned is required to observe high size variation. In previous studies, shrimp displaying WFS show a copy number in the order of ~ 106 copies/ng of extracted hepatopancreas DNA.
The reproduction of WFS in shrimp infected with EHP and challenged with V. parahaemolyticus is very likely due to the synergistic actions of the microsporidian and a particular bacterial strain on hepatopancreatic tubules. As mentioned previously, Huang and colleagues suggested that WFS is caused by intestinal microbiota dysbiosis and this proposition bears some similarities to our finding. Our observations during the early stage of EHP infection in shrimp displaying WFS include high lipid content in the hepatopancreatic tubule but some hepatopancreas cells being heavily infected. However, at this point, there is no obvious cellular shrimp immune response such as inflammation or hemocytic congestion. Later, as the infection progresses, EHP-infected epithelial cells in the HP tubule slough and expose the underlying basal membrane of the tubule to opportunistic bacteria that rapidly colonizes the affected tubules.
Bacterial colonization then leads to a cell response of the host resulting in hemocytic inflammation, subsequent melanization [pigmentation] and further sloughing. There is a moderate to severe atrophy of the hepatopancreas tubule cells leading to a partial or total dysfunction of the hepatopancreas. In a healthy shrimp, the bulk of the feces generated upon digestion of feed consists of residual vacuoles from hepatopancreas cells, which involve a major process for packaging and removing waste products from the digestive system. In EHP-infected shrimp, when the cluster of hepatopancreas cells are released from the tubule to the GI tract, the combination of lipids, digested/undigested feed, EHP and the bacterial mass will contribute with the formation of a whitish fecal string. It is possible, due to the presence of lipids in the feces, that the fecal mass becomes lighter compared to feces from a healthy shrimp and floats on the water surface water instead of sinking to the pond bottom.
Alterations involving the gastrointestinal tract, where a sign of the disease is whitish stools, are common to all animals including humans. In humans, whitish stools are caused by changes in the gut microbiome or by particular pathogens such as Cryptosporidium spp. This might also be the case for shrimp WFS, where the disease – and therefore the particular signs – are caused by two distinct etiologies. Our study clearly shows that the two pathogens induce white feces in shrimp and decisively shows that EHP plays a central role in the development of this syndrome, as our research group proposed in previous research.
Our study provides conclusive evidence that EHP is not the only pathogen associated with the presence of White Feces Syndrome. In Thailand, some studies have shown the association of WFS in shrimp population with an increase of Vibrio spp. where the intestines in shrimp, with and without WFS, were analyzed. An increase of intestinal Vibrio population from a normal shrimp with 1.8×107 CFU [colony forming units]/g population versus WFS-shrimp with 6.1×107 CFU/g. Some of these Vibrio. spp found in those samples were V. parahaemolyticus, V. vulnificus and V. damsela. This corroborates our identification of a strain of V. parahaemolyticus as the unidentified pathogen co-infecting with EHP on shrimp manifesting WFS. However, it is possible that other Vibrio species in addition to V. parahaemolyticus could induce WFS.
Conclusions
EHP is a primary enteric pathogen of penaeid shrimp. Infection with EHP in association with a particular strain of V. parahaemolyticus leads to the development of WFS. These findings also corroborate and provide an explanation as to why WFS is manifested in areas where EHP and V. parahaemolyticus are also endemic.
Now that you've reached the end of the article ...
… please consider supporting GSA’s mission to advance responsible seafood practices through education, advocacy and third-party assurances. The Advocate aims to document the evolution of responsible seafood practices and share the expansive knowledge of our vast network of contributors.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year.
Not a GSA member? Join us.
Authors
-
Luis Fernando Aranguren Caro, Ph.D.
Corresponding author
Aquaculture Pathology Laboratory
School of Animal and Comparative Biomedical Sciences
The University of Arizona
Tucson, Arizona, USA -
Hung N. Mai, Ph.D.
Aquaculture Pathology Laboratory
School of Animal and Comparative Biomedical Sciences
The University of Arizona
Tucson, Arizona, USA -
Roberto Cruz-Florez, Ph.D.
Ensenada Center for Scientific Research and Higher Education (CICESE)
Baja California, Mexico -
Frances Laureen Agcalao Marcos
Aquaculture Pathology Laboratory
School of Animal and Comparative Biomedical Sciences
The University of Arizona
Tucson, Arizona, USA -
Rod Russel R. Alenton, Ph.D.
Aquaculture Pathology Laboratory
School of Animal and Comparative Biomedical Sciences
The University of Arizona
Tucson, Arizona, USA -
Arun K. Dhar, Ph.D.
Aquaculture Pathology Laboratory
School of Animal and Comparative Biomedical Sciences
The University of Arizona
Tucson, Arizona, USA
Tagged With
Related Posts

Health & Welfare
EHP a risk factor for other shrimp diseases
Laboratory challenges and a case-control study were used to determine the effects of EHP infection on two Vibrio diseases: acute hepatopancreatic necrosis disease (AHPND) and septic hepatopancreatic necrosis (SHPN).

Health & Welfare
Challenging Pacific white postlarvae with AHPND
Study results indicate that P. vannamei challenged with AHPND in biofloc had higher survival rates than shrimp challenged in clear water.

Health & Welfare
New management tools for EHP in penaeid shrimp
Authors examined the histological features from shrimp infected with the emerging microsporidian parasite Enterocytozoon hepatopenaei (EHP). A PCR assay method was used to detected in hepatopancreatic tissue, feces and water sampled from infected shrimp tanks, and in some samples of Artemia biomass.

Health & Welfare
White Feces Syndrome in shrimp: Predictor of EHP?
Study demonstrates strong association between White Feces Syndrome and Enterocytozoon hepatopenaei in EHP-endemic regions. Biosecurity strategies can minimize the risk of pathogen’s spread in the Americas.



