Clear protocols needed for administration of live bacteria

Microbial problems are a major cause for low predictability in the production of juvenile Atlantic cod (Gadus morhua), as well as juveniles of most marine fish. Bacteria enter the fish larvae via the eggs, water, and live feed. As intensive culture conditions can be stressful for the early larval and juvenile stages of fish, pathogenic species are given excellent opportunities for proliferation and infection of the fish larvae.
At some hatcheries, antibiotics have been added to control bacterial growth, even though this strategy involves a high risk for the development of antibiotic resistance. Instead, measures for microbial control based on sustainable, environmentally friendly principles should be implemented to reduce or eliminate microbial problems.
Probiotic bacteria
The addition of probiotic bacteria to marine larvae is one tool that can contribute to reducing the proliferation of opportunistic and pathogenic bacteria and subsequent infection by establishing a protective normal flora on the mucosal surfaces of the fish larvae.
However, there are uncertainties regarding what properties the bacteria should possess and where to find the right candidates. Host-microbe interactions must be understood to establish the criteria used in the screening for probiotic bacteria.
Screening strategy
Due to the high numbers of bacteria candidates available, the authors screen potential probiotic bacteria by applying high-throughput strategies and robots in the search for probionts to be used in the first feeding of Atlantic cod larvae. The high capacity of the robots makes it possible to isolate and characterize many isolates within a few days, securing high diversity throughout the screening and reducing the risk of losing promising candidates at an initial stage.
The most important criteria in the selection are for the probiotic bacteria to participate in the normal gut flora and inhibit unwanted bacteria. In a study by the authors, both wild and farmed cod of different sizes were used for isolation of gut bacteria, resulting in a collection of approximately 5,000 isolates to be screened for probiotic characteristics. The robot transferred the isolates to marine broth added to 40 percent glycerol in plates stored at minus-20 degrees-C.
Various tests were adapted for the robot to characterize the most important traits of the isolates and group the strains. In addition to the enormous capacity, the automatic handling of the isolates reduced the human factor and resulting variance in testing. Classical phenotypic tests as well as tests for the characterization of antagonistic activity, haemolytic activity, bile tolerance, enzyme activity, and properties in mucus were adapted to the high-throughput screening.
Adhesion to and growth in gut mucus are important probiotic traits, as probionts must colonize the gut mucosa to become part of the normal intestinal flora in the fish larvae. A new method for screening strains based on their performance in cod gut mucus permitted the selection of strains in which more than 30 percent of the bacteria adhered to the mucus.

In vivo testing
The ultimate testing of the selected strains has to be done in feeding experiments with fish larvae. Such experiments are laborious, expensive, and require a high number of fish, so the number of trials should be kept to a minimum. The strength of robotic screening is that candidates can be screened for a wide range of probiotic properties to reduce the number tested in the labor-intensive experiments with fish larvae and juveniles.
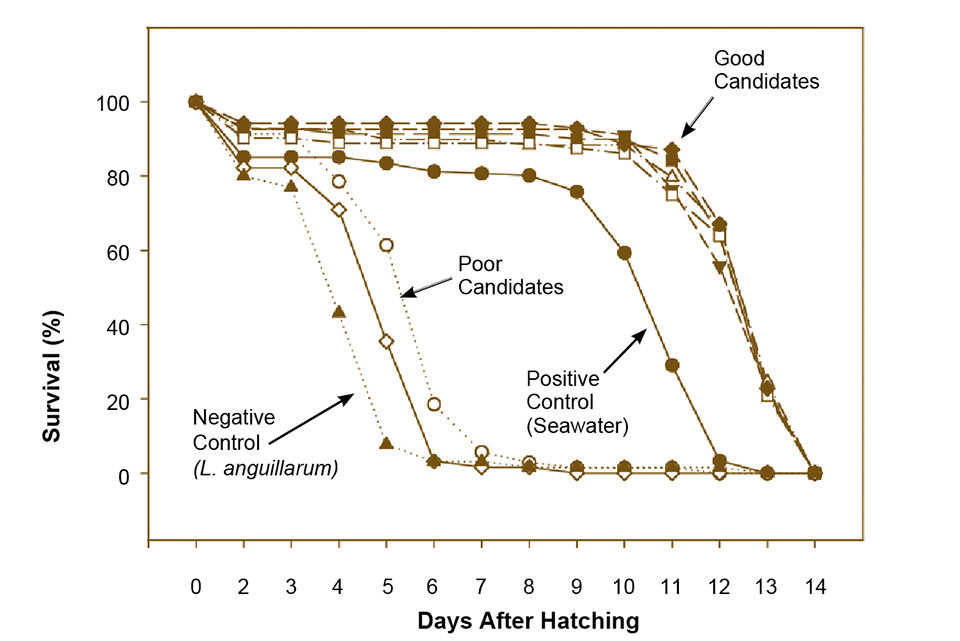
Before running first-feeding experiments, it is necessary to test probiotic candidates for eventual virulence against the fish larvae. The authors ran this type of test on cod yolk sac larvae hatched individually in plates for tissue and cell culture. Seven bacteria were added to the water in two different concentrations, and a known pathogen – the vibriosis-causing Listonella anguillarum – was used as a negative control, while nonsterilized seawater was used as a positive control.
Of the seven tested candidates, two were almost as virulent to the cod larvae as L. anguillarum, despite being both good antagonists and mucus-adhering bacteria. Five bacteria increased survival compared to the control and were characterized as very good probiotic candidates (Fig. 1). Additional work will concentrate on further testing of these five bacteria to generate enough documentation to ensure that they are true probiotics with no harmful effects to fish larvae and are safe for use in cod hatcheries.
Protocols needed
The use of probiotics in hatcheries can be challenging, so clear protocols for administration of the live bacteria are needed. The mucosal surfaces of fish larvae are colonized gradually, and the probiotic bacteria should be administered at early larval stages to increase the probability for successful establishment of a beneficial gut flora. The easiest method is addition to the water, but it may be more beneficial to use live feed organisms such as rotifers or artemia as vectors.
Since the detrimental bacterial flora of live feed cultures can effectively be exchanged with probiotic bacteria in a short-term enrichment stage, treatment of live feed with probiotics can introduce the beneficial bacteria at the same time the input of harmful bacteria is reduced. However, the stability of ingested bacteria in live feed can be rather low, so feeding protocols should ensure that the enriched organisms are eaten within a short time after feeding to the larvae.
Another way of administration is addition in formulated diets, either in premixes or as a coating, depending on the bacteria’s tolerance to heating and drying. This is an important subject for further research.
(Editor’s note: This article was originally published in the September/October 2007 print edition of the Global Aquaculture Advocate.)
Now that you've reached the end of the article ...
… please consider supporting GSA’s mission to advance responsible seafood practices through education, advocacy and third-party assurances. The Advocate aims to document the evolution of responsible seafood practices and share the expansive knowledge of our vast network of contributors.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year.
Not a GSA member? Join us.
Authors
-
-
Dr. Anders Fjellheim
Department of Biology
Norwegian University of Science and Technology
Trondheim, Norway -
Dr. Geir Klinkenberg
SINTEF Materials and Chemistry
Trondheim, Norway -
Dr. Inga M. Aasen
SINTEF Materials and Chemistry
Trondheim, Norway -
Dr. Olav Vadstein
Department of Biotechnology
Norwegian University of Science and Technology
Trondheim, Norway
Tagged With
Related Posts
Aquafeeds
Amino acid requirements in developing marine fish
Most marine fish have pelagic eggs with large pools of free amino acids of fairly constant proportion regardless of species. The amino acids provide energy for metabolism and are an important nutritional asset for growing embryos.

Health & Welfare
Barcoding, nucleic acid sequencing are powerful resources for aquaculture
DNA barcoding and nucleic acid sequencing technologies are important tools to build and maintain an identification library of aquacultured and other aquatic species that is accessible online for the scientific, commercial and regulatory communities.
Innovation & Investment
Better together: Partnerships drive innovation at leading labs
Laboratories with industry partnerships are making aquaculture more innovative, efficient and responsible. These collaborations offer access to expertise, facilities and funding to further the industry and improve global food security.
Health & Welfare
Do current shrimp practices favor EMS?
After refilling ponds, surviving microorganisms – including Vibrio parahaemolyticus, which causes EMS in shrimp – may benefit from the availability of nutrients in sediment and water and lack of competing microorganisms.


