Unreliable rotifer production remains a basic problem
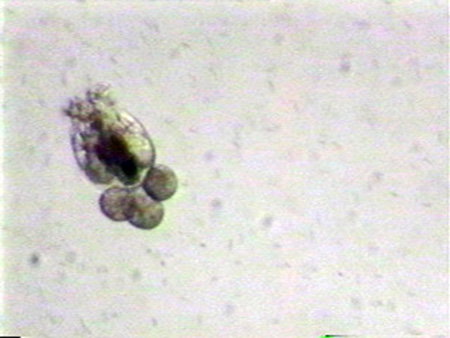
Successful mass culture of the marine rotifers Brachionus plicatilis (former L-type) and B. rotundiformis (former S-type) is one of the milestones of marine fish culture. Considerable research and subsequent manuals have been produced on the subject, but reliable rotifer culture remains a significant problem at many hatcheries. This is a stumbling block for mass production of juveniles of many species of marine fish. The application of basic management procedures can greatly facilitate consistent, reliable rotifer production.
Environmental conditions and life cycle
Rotifers are unique organisms with complex life cycles. Depending on environmental conditions, parthenogenetically (asexually-amictic) reproducing populations slowly fade into sexually producing (mictic) populations, with the occurrence of males, resting egg production, and an overall loss of production. Table 1 shows optimal culture conditions.
Culture temperatures above 30 degrees-C can lead to higher incidences of sexual reproduction and reduced levels of dissolved oxygen, especially at higher culture densities. Provision for pure or supplemental oxygen can be beneficial for stabilizing cultures at densities greater than 200 rotifers per millileter. Ammonia toxicity may not be a problem at pH levels below 7.5; regular water exchanges should be done to reduce ammonia levels.
Benetti, Optimal environmental conditions, Table 1
| Parameter | Preferred Range |
|---|
Parameter | Preferred Range |
|---|---|
| Temperature | 24-28° C |
| Salinity | 15-35 ppt |
| pH | 7.2-7.7 |
| Dissolved Oxygen | > 4 ppm |
| Light | > 2000 lux |
Keys to culture success
Rotifer life history and population dynamics are the driving factors of culture success. Similar to microalgae production, rotifer mass cultures exhibit the typical population cycle of an initial slow rate of increase or lag phase, a period of exponential increase or log phase, a transitional phase with little or no net change and a final declining phase.
Fecundity
Fecundity is probably the most important population parameter. It is expressed as the total number of eggs found on females, divided by the total number of rotifers, multiplied by 100 to express the value as a percentage. Monitoring fecundity allows for determination of the culture phase of the rotifer population. Highly fecund populations in their log phase are required for new starter cultures when upscaling cultures to larger mass-production tanks.
Density
Rotifer density is another critical quantitative variable that affects feeding regime and water quality. Besides the total number of rotifers, qualitative estimates of egg numbers per individual, gut content, activity, extent of detritus, and presence of contaminant organisms like ciliates should be recorded daily in a data sheet for each culture tank.
Inoculating new tanks
New culture tanks should be inoculated with initial rotifer densities above 50 per milliliter and fecundities as high as possible (minimum 30 percent). These populations should consist mainly of females carrying more than one egg. They can be produced by growing rotifers for 2 to 4 days in dense (greater than 106 cells per milliliter) microalgae cultures.
Feeding
Feeding regimes should follow a schedule where the higher the density of rotifers, the lower the amount of baker’s yeast or other food (Table 2).
When commercial formulated feeds are used, follow manufacturer recommendations, but adjust the application rate according to rotifer gut contents. Settling characteristics of artificial feeds should be considered. If continuous feeding is not possible, the total daily ration should be divided into four to six feedings spread evenly over the 24-hour period. Live microalgae should be supplemented if available, because they help maintain higher rotifer fecundities and growth rates.
Benetti, Rotifer density and amount of yeast, Table 2
| Density/million | Grams of yeast |
|---|
Density/million | Grams of yeast |
|---|---|
| < 50 | 3 |
| 50-100 | 2 |
| > 100 | 1 |
Avoiding crashes
Overfeeding and pollution of cultures due to uneaten food is one of the main reasons for culture crashes. Tank cleaning should be continuous, using suspended filter pads that can be removed and cleaned daily. In addition, the preferably round and conical tanks should be spun and settled daily for 15 to 30 minutes, with the settled detritus removed quickly via the drain or by carefully siphoning the bottom.
Planning production
Rotifer culture strategy has to consider that larval fish consumption of rotifers increases at the end of the two or three weeks the fish feed on rotifers. Consequently, rotifer harvest also has to increase just prior to the time when larvae are capable of ingesting and switching to artemia sp. nauplii as their main food item.
Full-time maintenance of cultures is required during the upscaling phase for some time prior to full production. Every hatchery should determine the number of rotifers and the volume of individual rotifer cultures to be maintained between larval production cycles, as well as the length of the buildup phase to full production levels.
Harvesting
Rotifers should be harvested by concentrating them in a harvester tank equipped with 44- to 70-μ mesh screen, depending on rotifer species and strain used. The screen should be submerged and self-cleaning, using air diffusers that brush the screen and prevent it from clogging with rotifers.
Rotifers should be harvested either by gravity or with a gentle pump that does not exceed 100 liters per minute. The harvester tank is also where the rotifers are thoroughly washed and rinsed to remove the dirty culture water, and concentrated prior to counting and adding to enrichment tanks.
Enrichment
Enrichment should be done overnight for at least 12 hours using nutritionally adequate and proven enrichment diets. After enrichment, rotifers have to be washed again. The presence of pathogenic bacteria associated with rotifers should be checked periodically by streaking them on TCBS-agar plates. The measurement of pathogens is especially relevant prior to the addition of rotifers to the larval fish cultures.
Feeding fish
Actual feeding of rotifers to fish should be the responsibility of the personnel who manage the larval fish. Automated delivery of live rotifers to larval fish tanks is preferred, with the daily ration of rotifers applied in multiple feedings. Automated feeding should be used to condition the fish larvae to the desired regime, and should continue through the periods of artemia nauplii feeding and weaning of larvae to artificial diets.
Conclusion
Although much information is available on mass culture of rotifers, unreliable rotifer production remains a basic problem in the successful production of marine fish juveniles. Following basic management procedures can improve the reliability and consistency of rotifer production.
Now that you've reached the end of the article ...
… please consider supporting GSA’s mission to advance responsible seafood practices through education, advocacy and third-party assurances. The Advocate aims to document the evolution of responsible seafood practices and share the expansive knowledge of our vast network of contributors.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year.
Not a GSA member? Join us.
Authors
-
Daniel D. Benetti, Ph.D.
University of Miami
Rosenstiel School of Marine and Atmospheric Science
Division of Marine Affairs and Policy
4600 Rickenbacker Causeway
Miami, Florida 33149 USA
-
Tagged With
Related Posts
Intelligence
A review of European marine finfish hatcheries
As European aquaculture continues to diversify in the marine fish sector, technology advances and methodology in marine finfish hatcheries are proving vital.

Health & Welfare
Achotines laboratory home to continuing studies of tuna early life history
The Inter-American Tropical Tuna Commission Achotines Laboratory in southern Panama is the world’s only facility with nearly year-round availability of tuna eggs and larvae. A study is comparing the reproductive biology, genetics and early life history of yellowfin and Pacific bluefin tuna.

Health & Welfare
Advances in yellowtail larval rearing
The University of the Canary Islands in Spain is researching yellowtail broodstock management and larval rearing to promote aquaculture diversification in Europe.

Health & Welfare
Bacterial testing improves biosecurity at marine hatchery
Microbial surveys of hatchery operations at Oceanic Institute found a strong correlation between hatchery success and cultivable bacteria levels in the water supplied to the larval-rearing systems.


