Overview of European, U.S. legislative systems

The regulation of antibiotic use has the objectives of guaranteeing the safety and efficacy of the drugs for the animals treated and protecting the health of consumers. As with other drugs, antibiotic use assumes the existence of some potential undesirable effects.
These can include the promotion of resistance to antimicrobial agents, interference with the normal microflora of the treated animals, residues in animal tissues destined for human consumption, potential toxic effects on the cells and tissues of animals, adverse effects due to interactions with other drugs or diseases, and allergic phenomena. Because of such effects, antibiotics are regulated by sanitary authorities.
European union legislation on antibiotics
In the European Union, the entity responsible for the evaluation of medical drugs is the Committee for Medicinal Veterinary Products (CVMP) of the European Agency for the Evaluation of Medicinal Products. Current legislation is based on Regulation no. 2377/90 of the council, dated June 26, 1990, and its later modifications.
Table 1 (right) lists the antibiotics that are authorized by the E.U. for use in aquaculture. Some are specifically authorized for fish species, while most are approved for use in all species destined for human consumption. Also included are prohibited antibiotics whose use is not authorized in any species destined for human consumption.
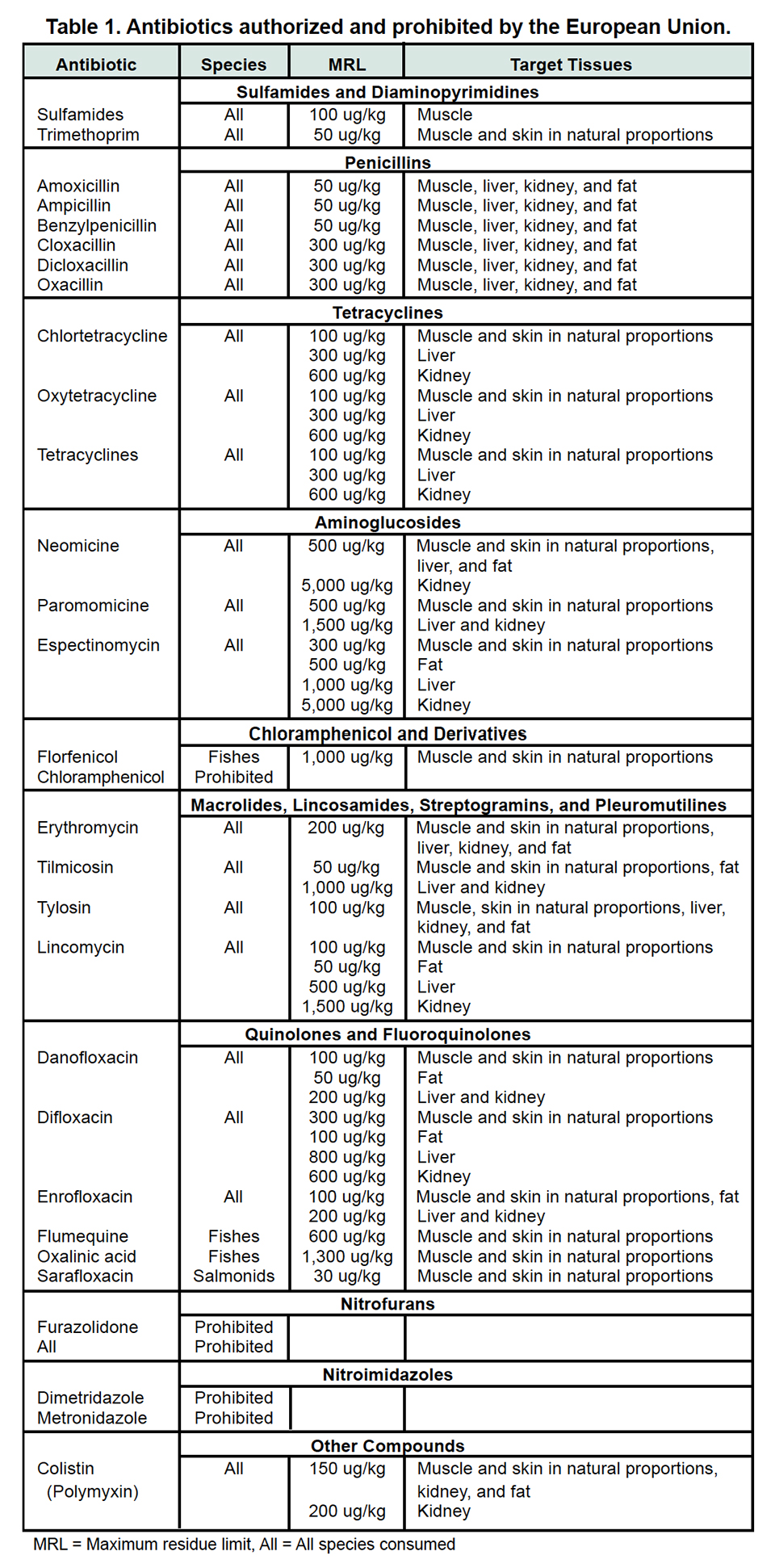
The CVMP document “Note for Guidance on the Risk Analysis Approach for Residues of Veterinary Medicinal Products in Food of Animal Origin” states that it could be possible to extrapolate the maximum residue limits for salmonids to all fish species.
U.S. use of antibiotics
The United States establishes norms, ensures they are followed and punishes infractions through the Food and Drug Administration (FDA). Through the Center for Veterinary Medicine, FDA is in charge of regulating the studies that pharmaceutical companies must present to obtain approval for drugs to be used in food animals.
This system contemplates the establishment of maximum levels for residues that are innocuous to consum-ers and the necessary requisites to establish the withdrawal period or waiting time between the administration of a drug to animals and its clearance from their systems.
The Food Safety and Inspection Service, under the U.S. Department of Agriculture, has the mission of national control of residue incidence through random sampling of tissues in slaughterhouses and their chemical analysis.
Table 2 lists the antibiotics prohibited by FDA for use in animals destined for human consumption. Table 3 lists the tolerated residue levels established by FDA for aquatic organisms.
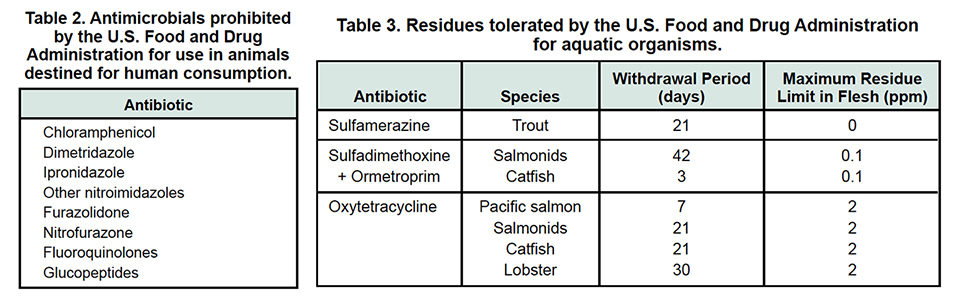
FDA’s title 21, chapter I, parts 500-600 code establishes the conditions under which specific antibiotics can be used in species for which they are not registered, with special emphasis on limitations for their applications in animals destined for human consumption.
For further information on antibiotic regulations:
Antibióticos para animales: Una perspectiva sobre antibióticos, salud animal y el debate sobre la resistencia. (1999) Federación Europea de la Industria de Sanidad Animal.
http://www.veterindustria.com/veter/temasdeinteres/docs/dossier1.pdf
EMEA/CVMP/342/99 Final Report: Antibiotic Resistance in the European Union Associated With Therapeutic Use of Veterinary Medicines. (1999) Report, qualitative risk assessment by Committee for Veterinary Medicinal Products.
European Agency for the Evaluation of Medicinal Products.
http://www.emea.eu.int/pdfs/vet/regaffair/034299ENC.pdf
Title 21: Food and Drugs. Chapter I. (April 2003) U.S. Food and Drug Administration, Department of Health and Human Services.
http://www.access.gpo.gov/nara/cfr/waisidx_03/21cfrv6_03.html
Versión consolidada de los Anexos I a IV del Reglamento no. 2377/90 delConsejo. (Julio 2003) Límites máximos de residuos de medicamentos veterinarios que pue-den aceptarse en alimentos de origen animal.
http://pharmacos.eudra.org/F2/mrl/conspdf/MRL%20consol%202003-07-22%20ES.pdf
Veterinary Medicines. (2003) European Agency for the Evaluation of Medicinal Products.
http://www.emea.eu.int/index/
(Editor’s Note: This article was originally published in the June 2004 print edition of the Global Aquaculture Advocate.)
Now that you've reached the end of the article ...
… please consider supporting GSA’s mission to advance responsible seafood practices through education, advocacy and third-party assurances. The Advocate aims to document the evolution of responsible seafood practices and share the expansive knowledge of our vast network of contributors.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year.
Not a GSA member? Join us.
Author
-
Victoria Alday de Graindorge, Ph.D.
INVE Technologies NV
Oeverstraat 7
B-9200 Baasrode, Belgium
Tagged With
Related Posts

Health & Welfare
Antibiotic-resistant bacteria, part 1
No antimicrobial agent has been developed specifically for aquaculture applications. However, some antibiotic products used to treat humans or land-based animals have been approved for use at aquaculture facilities.

Health & Welfare
Antibiotic-resistant bacteria, part 2
The development of antimicrobial resistance genes in human pathogens as a consequence of exposure to antibiotics in aquaculture is widely documented. Reports implicate foodborne antibacterial-resistant pathogenic bacteria in human disease.

Health & Welfare
Antibiotics in aquaculture: Is responsible use possible?
Regulations on antibiotics in aquaculture vary by country and region, from outright bans to minimal oversight.

Health & Welfare
A holistic management approach to EMS
Early Mortality Syndrome has devastated farmed shrimp in Asia and Latin America. With better understanding of the pathogen and the development and improvement of novel strategies, shrimp farmers are now able to better manage the disease.


