Challenge tests conducted at the Aquaculture Pathology Laboratory of the University of Arizona

Shrimp diseases, especially the viral diseases such as White Spot Syndrome Virus (WSSV) and Taura Syndrome Virus (TSV), have caused tremendous economic losses to the shrimp-farming industry and continue to threaten its long-term sustainability. After many years of research and practical experience, the industry has developed various management strategies to combat these diseases, ranging from implementing reliable diagnostic systems and applying chemical treatments to adopting biosecure production systems.
Most of these approaches to control shrimp disease, however, are costly and relatively ineffective as long-term solutions. The development of genetically improved shrimp strains resistant to viral agents is a long-term strategy that would help alleviate the industry’s problems.
In general, genetic improvement in shrimp production has lagged far behind the genetic progress achieved in other farm animals. But recently, selective breeding for shrimp stocks resistant to specific diseases received increasing attention. The Global Shrimp OP survey conducted by the Global Aquaculture Alliance concluded that the development of shrimp lines resistant to WSSV, TSV, Yellow Head Virus, and Infectious Hypodermal and Hematopoietic Necrosis Virus has become a high research priority.
Genetic selection program
In 2002, the SyAqua division of Sygen International launched a comprehensive commercial genetic selection program for the Pacific white shrimp (Litopenaeus vannamei) in one of its genetic nucleus breeding centers in Mazatlan, Mexico. The program targets a number of economically important traits and is based on a combination of family and individual selection. It benefits from the company’s extensive expertise in applying scientific principles of quantitative genetics and biotechnology to animal breeding, especially in pigs.
In 2003, a batch of 200 full-sib families was produced and raised in separate cages in the breeding center prior to individual tagging. Performance tests conducted in both the center and several commercial farms evaluated shrimp production traits such as growth rate and grow-out survival.
Of these 200 families, the 151 top families for hatchery performance were challenged with both TSV and WSSV. All information collected was integrated through a multitrait selection index aimed at simultaneously improving shrimp growth, survival, and disease resistance.
Disease challenge tests
All disease challenge tests were conducted at the Aquaculture Pathology Laboratory of the University of Arizona in Tucson, Arizona, USA. Via oral infection, Belize strain TSV (TSV-C), which has been shown more devastating than serotypes isolated from Hawaii and Mexico, and China strain WSSV were introduced to the shrimp.
Temperature testing
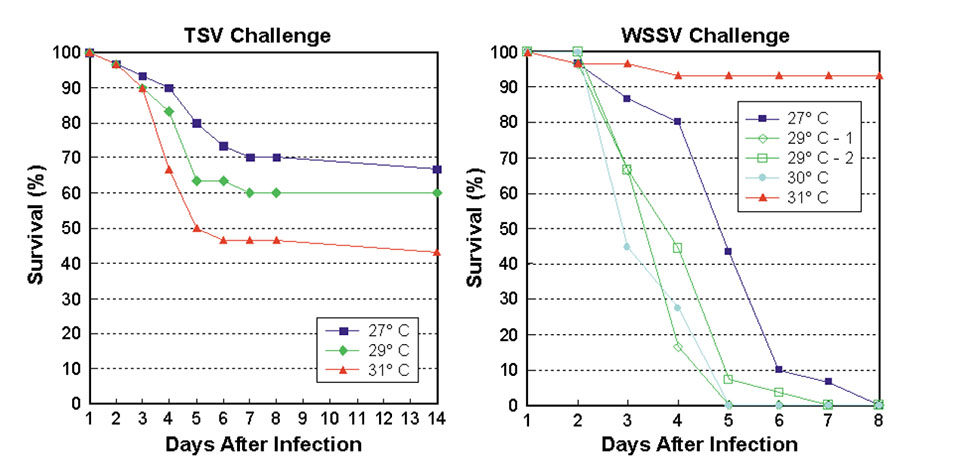
Previous research (Granja et al., 2003) has demonstrated the considerable impact of temperature on the survivability of L. vannamei exposed to lethal concentrations of TSV and WSSV. A preliminary trial was conducted to better quantify the effects of 27, 29, and 31 degrees C temperatures and optimize the testing protocol for family selection purposes.
The results of the preliminary trial (Figure 1) showed that in TSV challenge, mortalities occurred predominantly during the first week after infection and increased as temperatures rose from 27 to 31 degrees-C, with overall survival after 14 days of 57 percent. For WSSV challenge, a high survival rate was verified at 31 degrees-C. Below this temperature, shrimp died off faster as temperature increased. Almost all shrimp were dead by the end of the first week after infection, except those at 31 degrees-C.
Based on these findings, the family TSV and WSSV challenge tests were conducted at 30 and 27 degrees-C, respectively.
Two-phase challenge
The individual challenge tanks were terminated when their survival rates approached 50 percent, the theoretical threshold at which the largest variation between families is expected to be manifested. Tissue samples from all survivors were kept for molecular genotyping purposes, and routine random histological examinations were performed to monitor the initial health status of shrimp and confirm the observed mortalities were really due to the viral diseases under test.
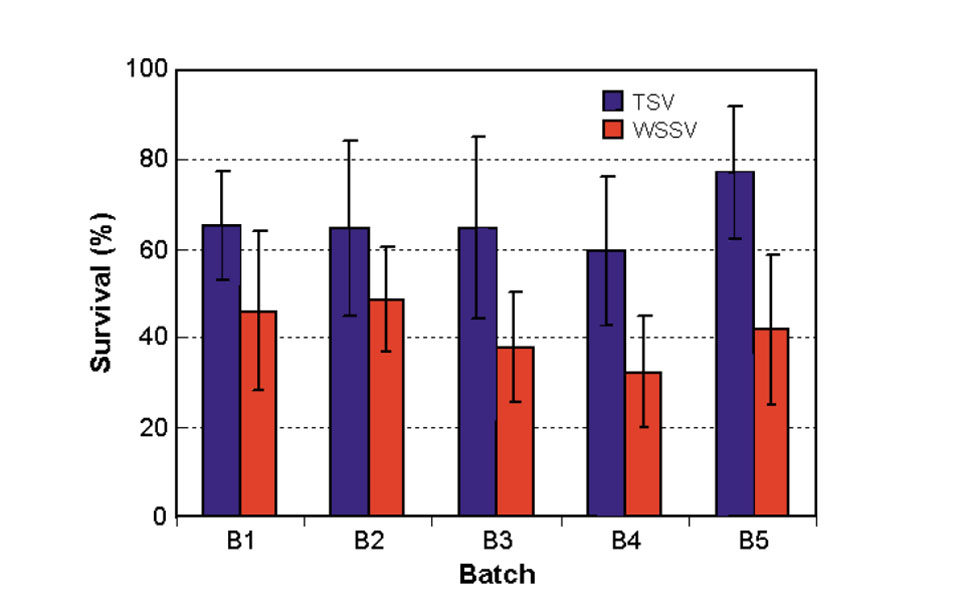
Two phases of challenge tests were conducted. In Phase I, 151 families divided into five batches were challenged for both TSV and WSSV. Average shrimp weight was 2.8 grams, with about 26 shrimp/family exposed to the viral agents. Positive and negative control tanks were maintained.
The negative control comprised a mixture of representatives from all families, while the positive control consisted of specific pathogen-free Kona stock L. vannamei obtained from the Oceanic Institute in Hawaii, USA. Since Kona shrimp are known to be very susceptible to these diseases, they were used to ensure the tissues fed to shrimp were infectious.
For TSV challenge, animals in the challenge tanks and positive control were fed minced tissues infected with TSV-C at a rate of 5 percent body weight/day for two successive days. For WSSV challenge, the challenge tanks and positive control received WSSV-infected tissues at the same rate, but on day 0 only.
The negative control was maintained using a commercial feed with 35 percent protein and 2.5 percent squid meal. Both the negative and positive controls were maintained for seven days, while the challenge tanks were terminated at 50 percent mortality, which usually occurred at days 3-4 for WSSV exposure. For TSV, the 50 percent mortality was often not reached at day 7, and by that time mortality had ceased.
Based on the results from Phase I, families with high or low resistance for each disease were identified and then rechallenged in Phase II to evaluate the repeatability of the disease challenge tests. The same experimental protocol was used in both phases. Ten families were selected for TSV and 18 families for WSSV rechallenge. Average shrimp weight in the Phase II rechallenges was 14 grams, with 28 shrimp challenged per family.
Results and implications
The results of the Phase I tests are presented in Fig. 2. A large between-family variation of 0 to 100 percent existed for survival rate after TSV-C exposure, with an average survival of 66 percent at the termination of the challenge tests. Relatively smaller (6 to 80 percent) between-family variation was found for survival after WSSV exposure, with an average survival of 42 percent.
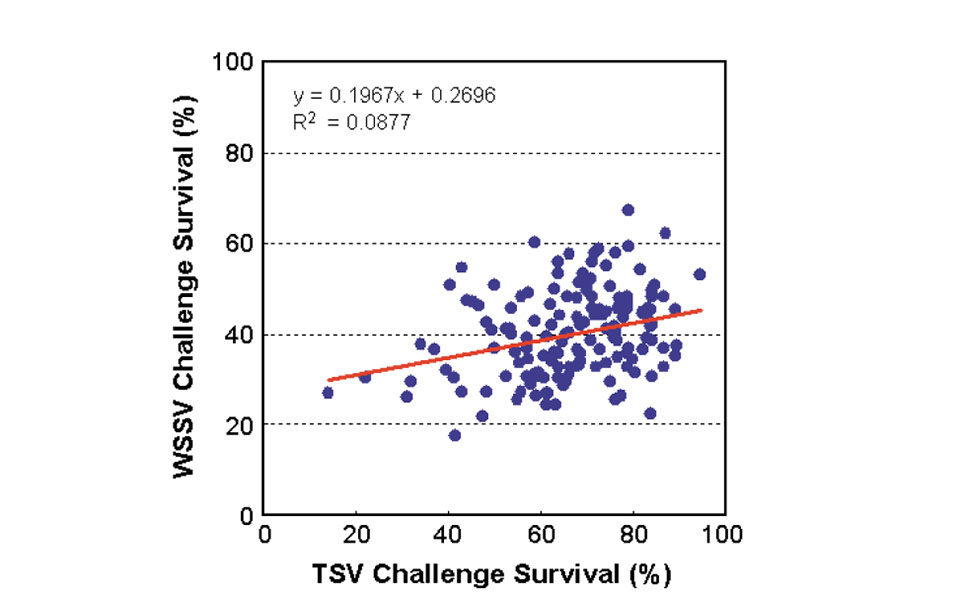
For the negative controls, survival rates were generally high at 82 to 100 percent. The positive controls had no survivors at the end of the TSV and WSSV challenge tests. Analysis showed a low but positive phenotypic correlation of 0.30 between family resistance to TSV and WSSV challenges (Fig. 3).
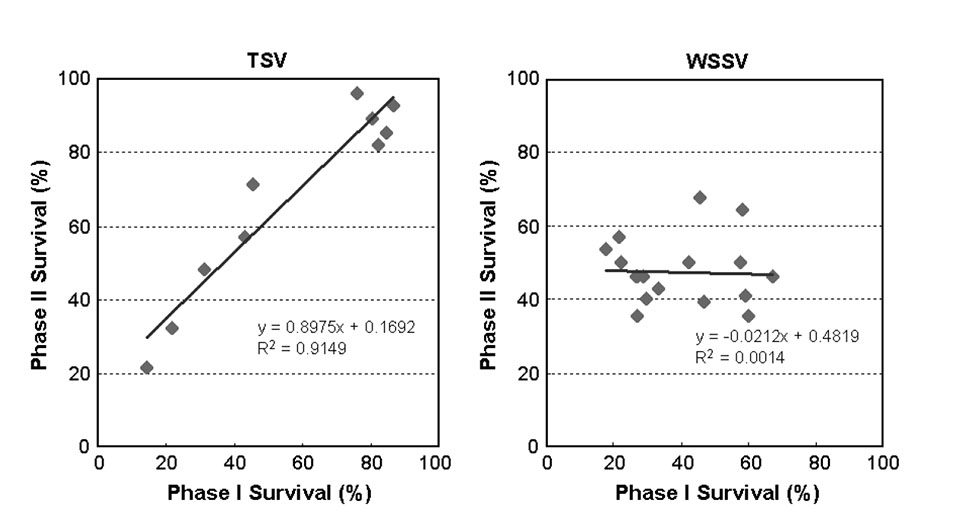
Comparing the survival rates of the families challenged in both Phase I and II (Fig. 4), the correlation of survival after TSV exposure between the two phases was very high (r = 0.96), while the correlation of survival after WSSV exposure between the two phases was rather low at r = 0.04. These results indicated that a large genetic variability for TSV resistance existed in the tested population.
Current challenge tests produce consistent and reliable results and are an effective tool for family selection. The resulting shrimp strains consistently exhibited over 90 percent survival in TSV challenge tests. In WSSV challenge tests, however, the low correlation between Phases I and II suggested the current testing methodology is less satisfactory, which might explain why no notable progress has been made in selecting for WSSV resistance using traditional approaches.
Clearly, a more specific test protocol needs to be developed for WSSV challenge tests to achieve consistent results. Quantitative and molecular genetic tools need to be developed and applied in order to improve the efficiency of selection.
Molecular approaches
Over the past two years, the Molecular Genetics Laboratory of Sygen has executed intense effort on genetic marker development through strategies that include comparative genomics, candidate gene approaches, and large-scale sequencing. Routine genotyping now involves a rapidly increasing number of markers.
The genotypes are statistically matched through appropriate association analyses with phenotypic data collected in the Sygen breeding program. The data includes results from the disease challenge tests, which allows the identification of markers associated with resistance to TSV or WSSV. These preliminary markers are then subject to further validation. Once confirmed for their disease-resistance effects, these genetic markers are integrated into the selection and mating strategies to increase the accuracy and intensity of selection.
To enhance its disease-resistance genetic marker development, a gene expression study is currently under way at Sygen that uses families with a large contrast in resistance for each disease. The objective is to more rapidly and precisely identify candidate genes for resistance to WSSV and TSV.
(Editor’s Note: This article was originally published in the February 2004 print edition of the Global Aquaculture Advocate.)
Now that you've reached the end of the article ...
… please consider supporting GSA’s mission to advance responsible seafood practices through education, advocacy and third-party assurances. The Advocate aims to document the evolution of responsible seafood practices and share the expansive knowledge of our vast network of contributors.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year.
Not a GSA member? Join us.
Authors
-
Donghuo Jiang, Ph.D.
Sygen International/SyAqua
2929 Seventh Street, Suite 130
Berkeley, California 94710 USA -
Joao L. Rocha, Ph.D.
Sygen International/SyAqua
2929 Seventh Street, Suite 130
Berkeley, California 94710 USA -
Daniel Ciobanu, Ph.D.
Sygen International/SyAqua
2929 Seventh Street, Suite 130
Berkeley, California 94710 USA -
Alan Mileham, Ph.D.
Sygen International/SyAqua
2929 Seventh Street, Suite 130
Berkeley, California 94710 USA -
Hein van der Steen, Ph.D.
Sygen International/SyAqua
2929 Seventh Street, Suite 130
Berkeley, California 94710 USA -
Brenda L. White-Noble
University of Arizona
Aquaculture Pathology Laboratory
Tucson, Arizona, USA -
Donald V. Lightner, Ph.D.
University of Arizona
Aquaculture Pathology Laboratory
Tucson, Arizona, USA
Tagged With
Related Posts

Health & Welfare
‘Big picture’ connects shrimp disease, inbreeding
Disease problems on shrimp farms may be partly driven by an interaction between management practices that cause inbreeding in small hatcheries and the amplification by inbreeding of susceptibility to disease and environmental stresses.

Health & Welfare
A comprehensive look at the Proficiency Test for farmed shrimp
The University of Arizona Aquaculture Pathology Laboratory has carried out the Proficiency Test (PT) since 2005, with 300-plus diagnostic laboratories participating while improving their capabilities in the diagnosis of several shrimp pathogens.

Health & Welfare
A holistic management approach to EMS
Early Mortality Syndrome has devastated farmed shrimp in Asia and Latin America. With better understanding of the pathogen and the development and improvement of novel strategies, shrimp farmers are now able to better manage the disease.

Intelligence
A land grab for salmon (and shrimp) in upstate New York
The operators of Hudson Valley Fish Farm see their inland locale as a pilot to prove that land-based fish farming, located in close proximity to major metropolitan markets, can be successful.


