Results showed the significant lytic effect of an endolysin on several strains of pathogenic Vibrio
Bacterial infections are among the most relevant problems associated with mass mortalities of fish larvae. In fish larvae, the main route of entry for bacteria is through live feeds, after the transition from endogenous to exogenous feeding. Rotifers (Brachionus plicatilis) are essential live prey in the larval rearing of marine fish species; however, rotifers can be major carriers of bacteria. Although most of these bacteria are not pathogenic per se, detrimental effects on fish larvae can be caused by the increment of these bacteria in rotifers.
Current techniques such as disinfection are not completely effective in achieving a complete bacteria-free marine hatchery environment. Furthermore, those treatments may lead to microbial imbalance, leaving an environmental niche wide open for the proliferation of opportunistic pathogens. Hence, innovative and environmentally friendly solutions to control vibrio infections in marine fish larvae are required. A feasible option to do so is the use of phage therapy or phage-endolysin tools.
Bacteriophages (viruses that infect and replicate within bacteria) have been proposed as antimicrobials against pathogenic bacteria in different fields, including aquaculture. Endolysins (also called lysins) are proteins produced by bacteriophages in the late stages of infection. These enzymes degrade components of the bacterial cell wall and eventually kill the infected bacterial host. Bacteriophage research has improved our knowledge about endolysins and their role in the bacteriophage lytic cycle, as well as applications of endolysins as antimicrobials.
Endolysins can be a promising antibacterial alternative to control bacterial infection in aquaculture. Their mechanism of action, based on hydrolysis of cell wall bonds, reduces the probability of resistance development and they are considered safe for organisms used as live feed, such as rotifers, as well as implying no risk for consumers’ health. Based on wide evidence that supports its effectiveness both in vitro and in vivo evaluations, endolysins have been recognized as promising antibacterial agents. However, their potential use in the aquaculture field has not been explored yet.
This article – summarized from the original publication (Romero, J. et al. 2024. Lysin and Lytic Phages Reduce Vibrio Counts in Live Feed and Fish Larvae. Microorganisms 2024, 12(5), 904) – presents the results of an assessment of the effectiveness of a lytic phage and endolysin to reduce the vibrio counts in both rotifers population and fish larvae. Our investigation aimed to explore the potential use of these biological agents in mitigating Vibrio bacterial loads, which pose a significant concern in the aquaculture industry due to their detrimental impact on the health and development of fish larvae.
Biotech-feed giant partnership to explore bacteriophage potential
Study setup
Our investigation aimed to explore the potential of biological agents such as the bacteriophage CH20 and the endolysin LysVPp1 in reducing Vibrio bacterial loads in both rotifer and fish larvae. Various Vibrio species were incorporated into this study, due to their capacity to induce diseases in multiple hosts, including fish, humans, and shellfish.
The studied endolysin LysVPp1 was synthesized by a commercial procedure and phage CH20 was isolated from mussel homogenate. One objective of the study was the evaluation of the lytic activity of endolysin LysVPp1 on Vibrio strains following an established protocol. The lytic activity assay was performed using a spectrophotometer.
The second objective was using the endolysin LysVPp1 and the phage CH20 to assess their efficacy in reducing the Vibrio load in rotifers. Rotifer cultures were prepared and all assays were performed in triplicate. The load of pathogenic Vibrio in both the rotifers and the water was measured by bacterial counts in TCBS (Thiosulfate–citrate–bile salts–sucrose agar is a type of selective agar culture plate that is used in microbiology laboratories to isolate Vibrio species) agar media.
The third objective was the evaluation of Vibrio reduction in a bioassay using larvae of zebrafish (Danio reiro) and an established protocol. The load of pathogenic Vibrio in both the fish larvae and the water was measured by bacterial counts on TCBS agar.
For detailed information on the experimental design, isolation of lytic phages and endolysin from selected Vibrio species, and all experimental procedures, bioassays and analyses refer to the original publication.
Results and discussion
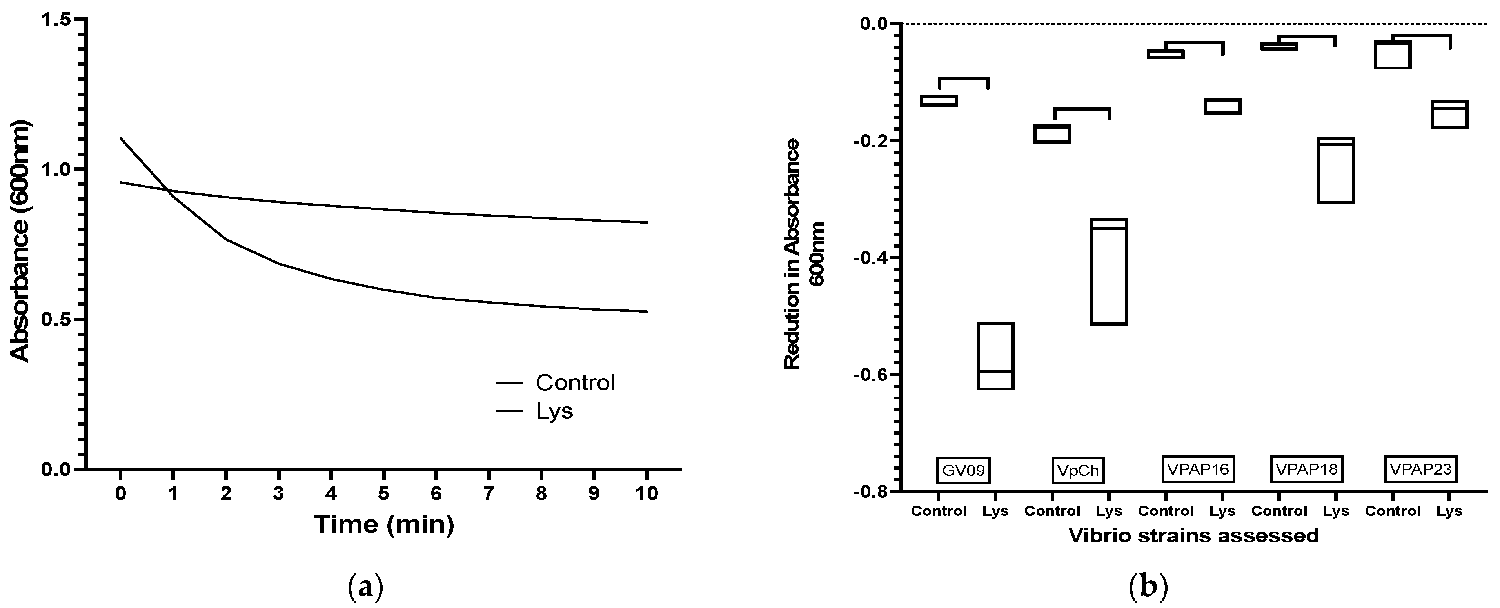
For the lytic activity of lysin against Vibrio strains, the spectrophotometer results revealed a notable decrease in absorbance values over time, indicating the lysis of Vibrio cells by lysin. For instance, the reduction in absorbance for strain GV09 is depicted in Fig. 1a, where a fast lytic activity was observed during the first 5 minutes of the assay. Additionally, the extent of reduction varied across different Vibrio strains evaluated, as illustrated in Fig. 1b, which demonstrates the reduction in absorbance for each evaluated Vibrio strain.
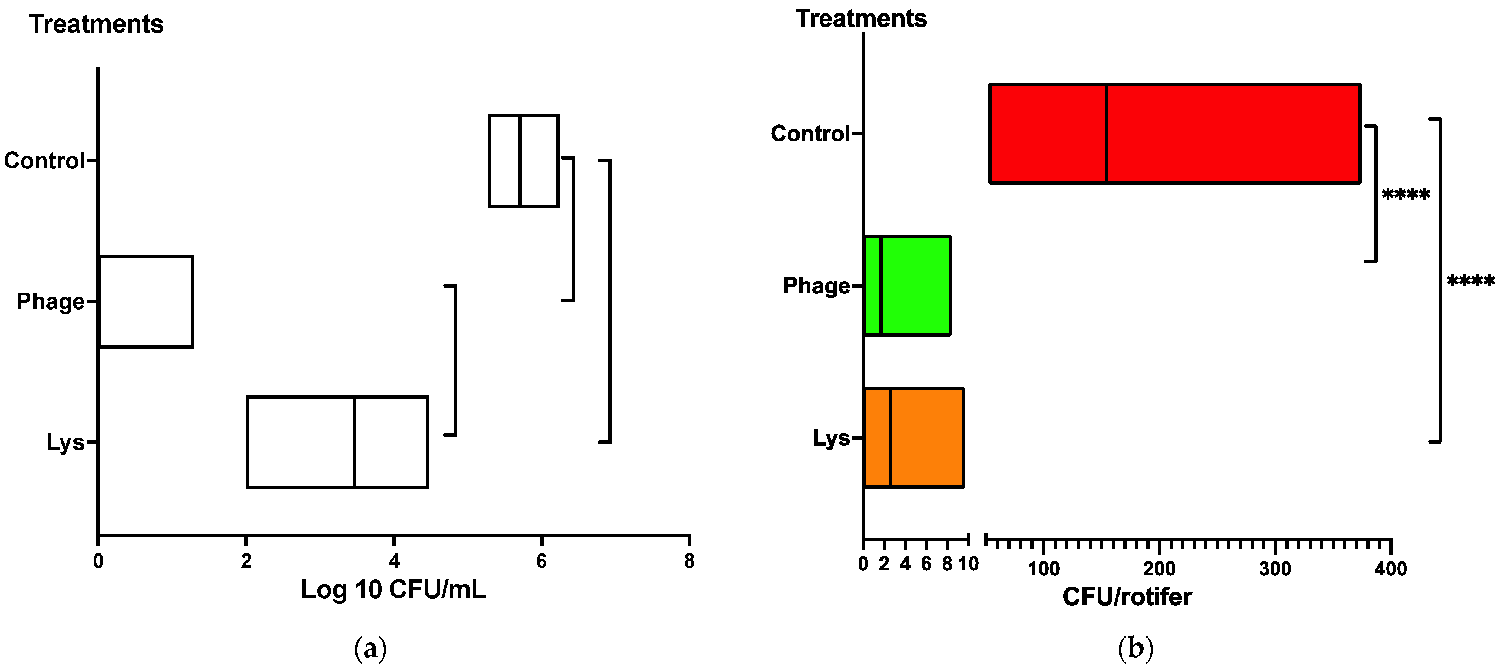
For the bioassay for the reduction in Vibrio load in rotifer, we evaluated the effectiveness of either LysVPp1 or CH20 in decreasing the counts of a specific Vibrio strain (GV09). The assay is tailored to last just 20 min, aligning with the swift requirements of potential applications in an aquaculture hatchery. The objective was to treat the rotifer swiftly to reduce Vibrio bacteria, and thus enhance its microbiological quality as a live food source for fish larvae. To better visualize the treatment effect, the reduction in Vibrio was measured both in the water and in the rotifers. In seawater, the reduction in Vibrio counts was notably significant following the phage CH20 treatment (Fig. 2). Similarly, with the endolysin LysVPp1, a substantial reduction was observed. Both treatments demonstrated statistically significant reductions in viable GV09 counts compared to the control group. Interestingly, phage CH20 caused a greater effect than endolysin in reducing Vibrio counts.
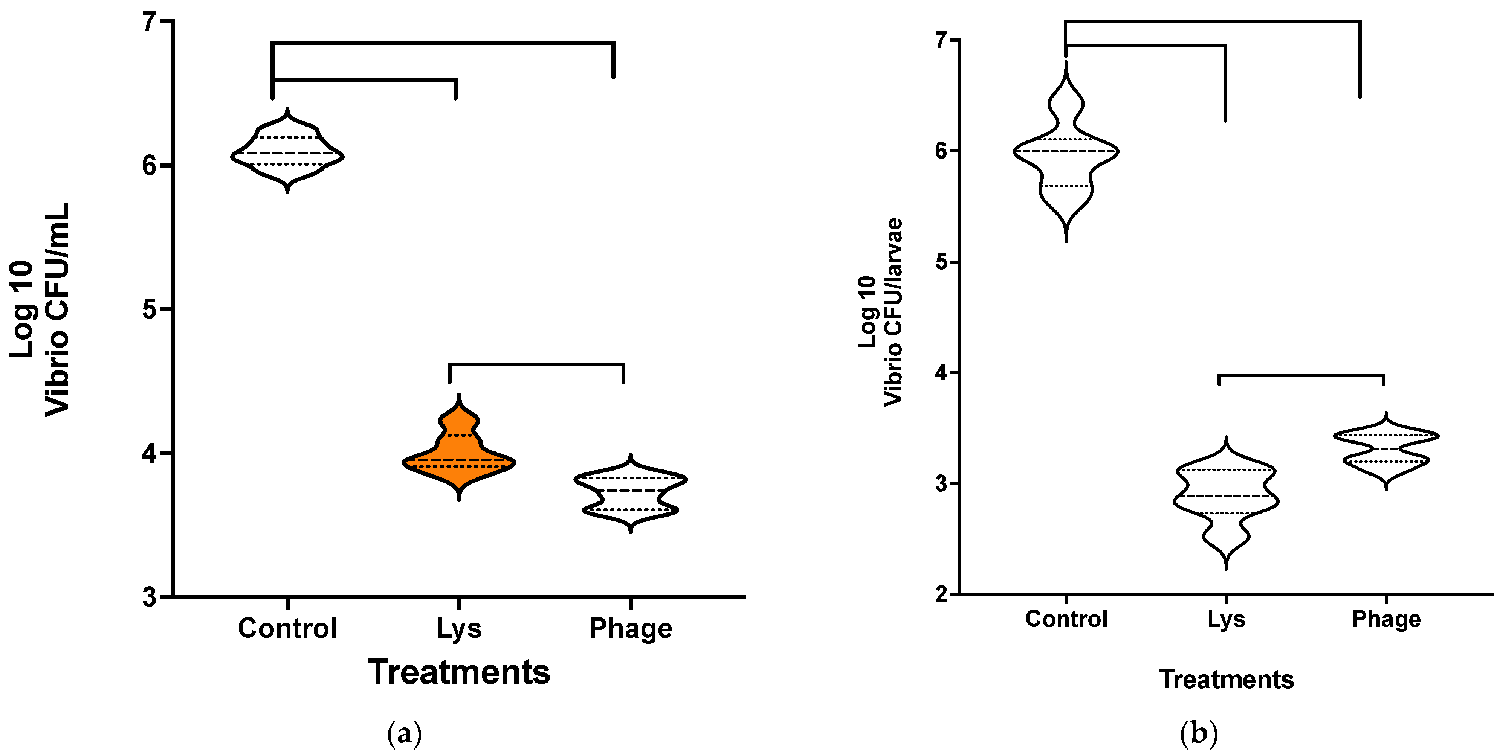
For the reduction in Vibrio load in fish larvae using a bioassay following the LysVPp1 treatment, the Vibrio count decreased significantly (Fig. 3): with the phage CH20, a significant bacterial decrease in the medium was also observed. Furthermore, both treatments demonstrated statistically significant reductions in viable Vibrio counts compared to the control group. Notably, the comparison of treatments showed that phage CH20 was notably more effective at reducing Vibrio counts, compared to endolysin.
The host range of CH20 was limited, in contrast to other Vibrio phages that exhibit a broad range of lytic activity. This narrow specificity is an advantage in the sense that phages will not disrupt host microbiota; however, it demands that each phage be screened against each bacterial target to determine susceptibility prior to treatment. Phage cocktails help mitigate the development of phage-resistant variants in bacteria and broaden the spectrum of bacterial strains that can be targeted. However, phage cocktails require the use of multiple phages, which can complicate the formulation and administration of the treatment.
Rotifers used as live feed host a wide array of microbiota. While these microorganisms are typically non-pathogenic to the feed organisms themselves, they can be passed on to larval predators and may cause harmful effects. The use of antibiotics was a straightforward solution to address these issues. However, their prophylactic administration led to several secondary effects, including an increase in the frequency of strains developing antibiotic resistance.
The significance of live feed – such as rotifers and artemia – as vectors in disease transmission has been emphasized due to the confirmed transfer of viruses to fish larvae. Applications of disinfection procedures, such as the ones successfully used in artemia hatching and enrichment, are lethal for rotifers. Consequently, numerous studies have been undertaken to diminish the bacterial load in rotifers before feeding them to fish larvae. The reductions in bacterial loads reported by various authors are limited compared to the phage or endolysin (LysVPp1) treatments, which significantly reduce the bacterial load in rotifers in a short time. This underscores the potential of phage or endolysin treatments in efficiently reducing bacterial contamination in rotifer cultures for aquaculture purposes.
The use of endolysins in fish larvae is a relatively new and developing area of research, and studies are being conducted to evaluate their efficacy, safety, and viability in different aquaculture systems. This approach is part of a broader effort to find alternatives to conventional antibiotics and to reduce the impact of bacterial diseases in aquaculture.
The use of phages or phage therapy has been studied in larvae; however, the focus was from the perspective of treatment, that is, reducing mortality, rather than reducing the levels of the pathogen, to prevent disease conditions. In the case of shrimp larvae, the early application of phage mixture (at 6 hours post-infection) was shown effective to support 80 percent survival). The use of endolysins for the control of early shrimp disease (AHPND), caused by Vibrio, is still in the evaluation stage of in vitro trials on the different strains reported to be associated with this disease.
Reducing the number of pathogenic or opportunistic bacteria as a strategy to prevent disease is a critical approach in various fields, including aquaculture. This strategy focuses on controlling the bacterial population to a level where it is less likely to cause infections or disease outbreaks. In aquaculture, treatments on larvae become operationally complicated, especially if there is a large volume to treat. Therefore, the focus is on treating the sources of pathogenic bacteria.
Among these, rotifers require gentle methods that do not damage their structure and allow for viability. By focusing on reducing the number of harmful bacteria, it is possible to maintain a healthier and more balanced environment, which is crucial for preventing the onset of diseases. This strategy is especially important in closed systems like aquaculture, where the spread of pathogens can be rapid and devastating.
Perspectives
Our findings provide compelling evidence for the viability of reducing the burden of pathogenic Vibrio in live feed and fish larvae through the utilization of antibiotic alternative tools, specifically lytic phages and endolysin Lys. The efficient and rapid action of these treatments highlights their potential for application in hatcheries. This promising aspect underscores the importance of exploring and implementing such strategies as part of sustainable aquaculture practices. By helping mitigate the presence of pathogenic bacteria in crucial stages of fish development, these interventions can contribute to enhanced health and welfare of cultured fish.
Now that you've reached the end of the article ...
… please consider supporting GSA’s mission to advance responsible seafood practices through education, advocacy and third-party assurances. The Advocate aims to document the evolution of responsible seafood practices and share the expansive knowledge of our vast network of contributors.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year.
Not a GSA member? Join us.
Author
-
Dr. Jaime Romero
Corresponding author
Laboratorio de Biotecnología de Alimentos, Instituto de Nutrición y Tecnología de los Alimentos (INTA), Universidad de Chile, El Líbano 5524, Santiago 7830489, Chile
Tagged With
Related Posts

Health & Welfare
A study of Zoea-2 Syndrome in hatcheries in India, part 1
Indian shrimp hatcheries have experienced larval mortality in the zoea-2 stage, with molt deterioration and resulting in heavy mortality. Authors investigated the problem holistically.

Health & Welfare
Designing a biosecurity plan for shrimp aquaculture, part 1
The development of an effective biosecurity plan requires full understanding of facility design and operations, and knowledge of the animals’ health status and the transmission modes of pathogens in order to identify the risks and define meaningful measures.

Innovation & Investment
‘Choices are limited when searching for alternatives to antibiotics’: How one veterinarian is employing bacteriophages to fight Vibriosis in shrimp farming
GOAL 2022: Bacteriophages can overcome antibiotics in aquaculture's fight against Vibriosis in shrimp farming, and Dr. C.R. Subhashini is leading that fight.

Health & Welfare
Potential applications of bacteriophages for AHPND control
Study demonstrates that isolated phages tested are effective in controlling AHPND infection in farmed penaeid shrimp and inhibiting bacterial growth.



