Peracetic acid does not react with proteins to produce toxic or carcinogenic compounds
The sources of spoilage and pathogenic microorganisms in fish- and shellfish-processing facilities include raw materials, workers, equipment, containers, floor drains, ventilation systems, and water applied under pressure during cleaning and sanitation procedures. Even when cleaning and sanitizing operations are regularly performed, not all microorganisms are eliminated from food and nonfood contact surfaces.
If microorganisms are not destroyed, they can grow during processing, distribution, retailing, and preparation, which reduces the quality of the product and can present a possible food safety hazard. The removal of contaminant microflora from surfaces in processing facilities can be achieved using different sanitizers.
Sanitizers and sanitation
The question of which sanitizer to use will depend on cost, availability, the nature of the soil in the facility, the processing equipment and facility materials, and the conditions under which food is processed. Sanitizer selection is made more difficult by the increased resistance to antimicrobials exhibited by adherent cells (biofilms) and the fact that information on the effectiveness of most sanitizers was obtained from tests on suspended planktonic cells.
When microorganisms settle on or adhere to a surface, they can be protected by irregularities in the surface that hamper the action of sanitizers. Therefore, the efficiency of sanitizers under specific application conditions must be well defined for effective sanitation programs to be implemented.
Peracetic acid
Peracetic acid possesses many advantages when compared to sodium hypochlorite, one of the most common sanitizers. One important advantage is that it does not react with proteins to produce toxic or carcinogenic compounds. It also has a low environmental impact, and has been reported more effective than sodium hypochlorite against biofilms.
Peracetic acid can be used over wide spectrums of temperature (0 to 40 degrees-C) and pH (3.0 to 7.5), in clean-in-place processes, and with hard water. In addition, protein residues do not affect its efficiency. However, it may not provide the microbial reduction sometimes achieved by sodium hypochlorite.
Poultry studies
Only limited research has been performed on the effectiveness of peracetic acid as a sanitizer in fish and shellfish processing. However, studies with other food products provide an excellent reference for what might be achieved with seafood.
In a 2004 study, three treatments – 30 milligrams per liter hydrogen peroxide, 0.5 percent peracetic acid, and 125 milligrams per liter ozone – and a chlorine control were applied to birds that were then sampled for the presence of Salmonella bacteria (Fig. 1).
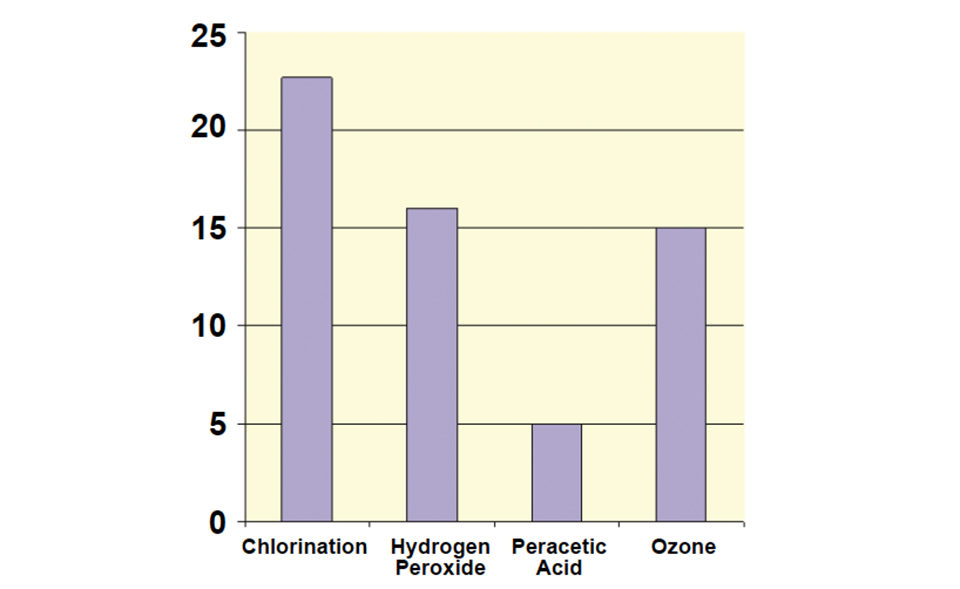
The bacterial load was significantly (P < 0.05) reduced after treatment with peracetic acid. The effectiveness of chlorine as a disinfectant was reduced when pH exceeded 6.0, temperature was below 30 degrees-C, and in the presence of some organic substances. The chlorine also led to the formation of biofilms, which exacerbated cleaning and sanitizing.
A second poultry study examined the populations of Campylobacter jejuni after exposure to water containing chlorine and peracetic acid (Table 1). Peracetic acid and chlorine were -equally effective, producing a 90 percent decrease in numbers when used at 100 ppm for 15 minutes of exposure, and no significant decrease when used at 40 ppm for two minutes.
Flick, Campylobacter jejeuni populations, Table 1
| Chemical | Concentration (ppm) | Live Cell Count (log CFU/cm2)* |
|---|
Chemical | Concentration (ppm) | Live Cell Count (log CFU/cm2)* |
|---|---|---|
| Control | 5.5 ± 0.6a | |
| Chlorine | 40 | 5.0 ± 0.1ab |
| 100 | 4.8 ± 0.5b | |
| Peracetic acid | 40 | 4.9 ± 0.5ab |
| 100 | 4.8 ± 0.1b |
Other studies
In another study, waters containing total coliforms, fecal coliforms, Eschericihia coli, and enterococci were treated with chlorine dioxide and peracetic acid. Results from the study (Table 2) showed that peracetic acid was as effective as chlorine dioxide in reducing total coliforms, fecal coliforms, and Escherichia coli. Chlorine dioxide was more effective in reducing the total plate count and enterococci count.
Flick, Escherichia coli populations, Table 2
| Reduction (%) Peracetic Acid | Reduction (%) Chlorine Dioxide |
|---|
Reduction (%) Peracetic Acid | Reduction (%) Chlorine Dioxide | |
|---|---|---|
| Heterotrophic total count at 36° C (CFU ml-1) | 80-28 | 85-77 |
| Total coliforms (MPN 100 ml-1) | 98-30 | 98-89 |
| Fecal coliforms (MPN 100 ml-1) | 99-01 | 99-20 |
| Escherichia coli (MPN 100 ml-1) | 99-45 | 99-97 |
| Enterococci (MPN 100 ml-1) | 91-29 | 98-64 |
Stainless steel plates containing 1 x 108 colony-forming units (CFU)/cm2 of Listeria monocytogenes and Pseudomonas sp. biofilms were subjected to a hypochlorite compound and peracetic acid at varying concentrations for one and five minutes (Table 3). There were no differences between residual Listeria populations, but some significant differences between residual Pseudomonas populations were observed. The differences, however, were probably not large enough to be of practical concern, since large reductions in the microorganism were achieved.
Flick, Effects of chlorine and peracetic acid, Table 3
| Microorganism | Sanitizer | Concentration (mg/l) and Exposure Time* 40 1 minute | Concentration (mg/l) and Exposure Time* 40 5 minutes | Concentration (mg/l) and Exposure Time* 80 1 minute | Concentration (mg/l) and Exposure Time* 80 5 minutes |
|---|
Microorganism | Sanitizer | Concentration (mg/l) and Exposure Time* 40 1 minute | Concentration (mg/l) and Exposure Time* 40 5 minutes | Concentration (mg/l) and Exposure Time* 80 1 minute | Concentration (mg/l) and Exposure Time* 80 5 minutes |
|---|---|---|---|---|---|
| Listeria | Peracetic acid | 4.0a | 3.6a | 3.8a | 2.7a |
| Chlorine | 5.4a | 3.2a | 3.6a | 2.8a | |
| Pseudomonas | Peracetic acid | 4.5b | 7.2a | 7.2a | 3.5a |
| Chlorine | 7.2a | 5.2ab | 5.6ab | 4.9a |
A disinfection study was performed on lettuce comparing the effectiveness of 80-ppm peracetic acid and 200-ppm sodium hypochlorite. The results showed that the effectiveness of peracetic acid was equivalent to that of sodium hypochlorite (Table 4). Both sanitizers were capable of effecting a 99 percent reduction in mesophilic plate count and total coliforms.
(Editor’s Note: This article was originally published in the June 2005 print edition of the Global Aquaculture Advocate.)
Now that you've reached the end of the article ...
… please consider supporting GSA’s mission to advance responsible seafood practices through education, advocacy and third-party assurances. The Advocate aims to document the evolution of responsible seafood practices and share the expansive knowledge of our vast network of contributors.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year.
Not a GSA member? Join us.
Author
-

George J. Flick, Jr., Ph.D.
Food Science and Technology Department
Virginia Tech/Virginia Sea Grant (0418)
Blacksburg, Virginia 24061 USA
Tagged With
Related Posts

Responsibility
Peracetic acid products expand sanitizing, organic water treatment options
Peracetic acid products, which have strong anti-microbial effects, can be used as sanitizers to control water quality in aquaculture systems.

Innovation & Investment
Developments in closed-containment technologies for salmonids, part 2
In the second of a two-part series, Steven Summerfelt discusses various efforts to improve knowledge of closed-containment systems, innovations in fish feed and presents comprehensive concluding remarks.

Responsibility
Hydrogen peroxide: Disinfectant for recirculating aquaculture systems
Hydrogen peroxide is a candidate for water treatment in aquaculture systems for it has antimicrobial effects and easily degrades to harmless byproducts.

Health & Welfare
Linking water treatment practices and fish welfare
Quantification of fish behavior is complex and depends on the experimental setup and biomarkers applied. A simple method was tested to quantify locomotor behavior to evaluate the effects of simulated water treatment scenarios using peracetic acid.

