Systems are simple, efficient, easy to use and low-cost

Amesocosm is an intermediatesized system that simulates the natural environment. In the world of marine fish hatcheries, it refers to tanks or small ponds of 10 cubic meters or larger in which a natural phytoplankton or zooplankton bloom is developed prior to stocking yolk-sac larvae.
Mesocosm systems have been traditionally used for semi-intensive larvalculture of red sea bream and several other marine fishes in Asia, particularly in Japan and Taiwan. They were successfully used in European hatcheries for the production of sea bream, sea bass, and turbot. Semi-intensive systems were also employed in Ecuador for raising Pacific yellowtail, a pelagic species from the southwestern Pacific. A variation of this method was applied in the United States for experimentally raising species such as red drum, red snapper, snook, and southern flounder.
Although they have proven technologically feasible and a viable alternative for the commercial production of high-value marine food fish in hatcheries, there is no standard protocol for raising marine fish larvae using mesocosm systems. The following is a general protocol for tank systems.
Tank preparation
Approximately one week prior to stocking, tanks are washed and scrubbed using a 10 percent muriatic acid solution, then chlorinated at 50 ppm for 24 hours and neutralized with sodium thiosulphate solution. Any other efficient cleaning and disinfecting method can also be used. Tanks should have an internal standpipe wrapped in 300-μ filter mesh to allow water exchange and prevent larvae escape.
Chlorinated and filtered seawater or unfiltered seawater can be used. The former allows for more control, whereas the latter is less labor-intensive, since it uses seawater containing a high diversity of phytoplankton and zooplankton pumped directly from the source. Tank water is fertilized with mixed nutrients to stimulate an algal bloom. A complete algal micronutrient mix, such as Guillard’s F/2 formulation, can be added to nutrient-poor water. Or a simple mixture of nitrogen, phosphorous, and potassium can be added to nutrient-rich water.
Microalgae
Any available microalgae species with adequate nutritional profile can be inoculated alone or in combination with other species in the tanks to create a bloom. A 1:1 combination of Nannochloropsis sp. and T. Isochrysis, along with other naturally occurring phytoplankton (primarily diatoms), will likely work well in the tropics. Depending on the predominant species, water color may turn dark green, golden brown, or anything in between.
Probiotics
Commercially available yeast-based products containing mainly Bacillus sp. can be added at 10 ppm to the system before stocking larvae and periodically thereafter. If filtered and chlorinated seawater is used, wild phytoplanktonicand zooplanktonic organisms must be stocked after the tanks are filled. This will require a source of microalgae and the previous identification of a source of zooplankton.
Wild zooplankton can be collected by towing a plankton net through coastal water. For small-size larvae – typical of most marine fish species – plankton should be filtered with 100-μ mesh, and only the smaller organisms should be used for first feeding.
Artificial substrates

Artificial substrates that mimic seagrass beds can improve production by improving water quality, supplementing natural foods, and reducing stress. They provide a surface for attachment of algae and nitrifying bacteria to reduce levels of NH4. They also act as a natural habitat for larvae and food organisms.
A wide variety of bacteria and other beneficial periphyton organisms typically colonize the artificial subtropical strates, creating a complex colony of microscopic algae, small crustaceans, copepods, rotifers, polychaetes, nematodes, and others. Dimensions of the artificial substrates depend on tank size. Substrates can float or be suspended from the top, but they are preferably weighted down with sandfilled PVC pipes to stay at least 50 mm off the bottom to allow proper water circulation.
Water exchange and aeration
Water exchange rates vary widely according to the density of the plankton bloom and the biological load. Water quality parameters must be monitored daily. Total ammonia nitrogen levels may rise quickly in an unbalanced, poorly managed system. Low water-exchange rates of approximately 10 percent per day are recommendedinitially, with progressive increases to 100 percent daily exchange during the third and fourth weeks and prior to metamorphosis.
Air stones should be evenly distributed around the tanks to provide moderate aeration. Air- or water-powered protein skimmers should be used to control organics and fine suspended solids. Any changes in water quality should be implemented slowly and over extended periods of time. The system has to be managed to keep water quality parameters within ideal ranges while keeping phytoplankton and zooplankton blooms stable for long periods of time.
Larvae stocking and plankton management
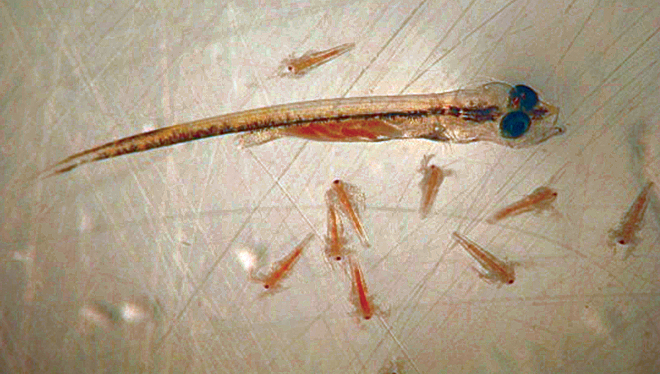
Stocking newly hatched larvae rather than fertilized eggs is preferable, to avoid the large amount of organics derived from egg shells and unhatched eggs. Yolk-sac fry can be stocked at relatively low densities (e.g., 4 to 12 per liter, or 80,000 to 200,000 per 20-t tank). A highly diverse assortment of zooplanktonic organisms in a variety of sizes – most likely consisting of copepods and other crustaceans – will likely bloom in the tank and become available as prey to the larvae.
Blooms initially occur in small pockets lining the perimeter of the tanks, and spread throughout the entire system at concentrations greater than 10 organisms per milileter before tapering off two weeks after the initial introduction of copepods. Plankton blooms can be routinely maintained for several weeks through subtle manipulation of shading, water flow, aeration, and the addition of microalgae.
Visual measurement of water turbidity using a Secchi disc in a “seasoned” mesocosm system could yield average readings of 50 cm, but would widely range among the tanks and even within the same tank over short periods of time. It is not uncommon to observe blooms of other zooplankton organisms, including jellyfish and other potentially dangerous species that could prey upon or compete with the fish larvae. Rotifers and artemia can be added as needed to supplement the natural productivity of the tanks and keep the concentration of live organisms at desirable levels of 0.1-10 per milliliter.
Advantages
Mesocosm systems generally compare favorably to traditional intensive larval husbandry techniques, although survival rates of marine fish larvae may be lower in these systems. Rates of growth and development are significantly higher in outdoor, semi-intensive mesocosm systems than in indoor intensive systems. Mesocosm systems offer greater environmental stability by preventing rapid, drastic changes in water quality due to the larger volumes used. They also tend to be less expensive to operate, due to greater reliance on natural foods, instead of artemia and formulated feeds.
Disadvantages
Mesocosm systems currently lack production consistency and a protocol that could be applied to different species in different regions – a paramount hurdle, since phytoplankton and zooplankton organisms vary widely from place to place, as do the nutritional and environmental requirements of the species raised. The systems also require large areas, but this is not a problem where land is inexpensive and readily available.
Conclusion
With much progress in recent years, the mesocosm method has been routinely incorporated into commercial marine fish hatcheries throughout the world. Many commercial hatcheries in Asia and Europe currently rely on mesocosm systems for their simplicity, efficiency, relative ease of use, and reduced cost and labor requirement.
The greatest challenge for successful production of marine fish fingerlings using the mesocosm method is to keep water quality parameters at ideal ranges, while maintaining phytoplankton and zooplankton blooms stable for long periods of time. This requires hard work, experience, knowledge and, above all, common sense.
The following contributed to this article: Michael W. Feeley, Owen Stevens, Scott Zimmerman, Jorge Alarcón, Yoichi Minemoto and Brian O’Hanlon.
(Editor’s Note: This article was originally published in the February 2001 print edition of the Global Aquaculture Advocate.)
Now that you've reached the end of the article ...
… please consider supporting GSA’s mission to advance responsible seafood practices through education, advocacy and third-party assurances. The Advocate aims to document the evolution of responsible seafood practices and share the expansive knowledge of our vast network of contributors.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year.
Not a GSA member? Join us.
Author
-

Daniel D. Benetti, Ph.D.
University of Miami
Rosenstiel School of Marine and Atmospheric Science
Division of Marine Affairs and Policy
4600 Rickenbacker Causeway
Miami, Florida 33149 USA
Tagged With
Related Posts

Health & Welfare
Bioreactor technology for tilapia advances in Latin America
Early biofloc-based culture systems in tanks in Brazil have given way to commercial-scale bioreactors housed in greenhouses that utilize water fertilization regimes, high-protein feeds and salinity controls.

Health & Welfare
Integrated spotted red snapper aquaculture in Central America
The technology for closing the life cycle of spotted red snappers has progressed due to efforts of government and private-sector researchers.
Aquafeeds
Live diets for larval fish, shrimp: Production, enrichment, feeding strategies
Live diets for reared marine larvae must be cost-effective and versatile while providing good nutrition and being easily captured and digested. Copepods offer superior nutritional value, but their rearing requires space and is laborious.

Aquafeeds
Novel reactor developed for indoor, high-density production of diatoms
The development of this reactor for the indoor cultivation of non-suspended microalgae like important diatoms such as Amphora spp., and the cellular dry matter values produced in this study will help bio-filming science support the development and improvement of in situ feed supplementation for fish and shrimp ponds, particularly in desert environments.

