Colorimeters evaluate shrimp raised on colored substrates
Color can significantly impact the price of shrimp, with darker-red and consistently colored shrimp preferred in a number of global markets. Such shrimp attract premium prices up to $4/kg higher than paler animals.
As part of Australia’s Commonwealth Scientific and Industrial Research Organisation (CSIRO) Food Futures Flagship initiative to improve shrimp coloration and maximize market value, the authors have adapted the use of colorimeters to measure and quantify color in shrimp.
Colorimeters
Colorimeters are normally used to measure the colors of paints and plastics. They are also used routinely in the meat and livestock industries to quantify meat quality attributes. Colorimeters provide reliable and objective measures of color, rather than the subjective color grade scoring that is currently used by the shrimp industry to estimate color.
Although using the machines requires some technical skill, animals can be measured raw or cooked, and the machines take the guesswork out of measuring shrimp color.
A number of portable colorimeters have been developed. In each case, color is measured using a standardized light source from a constant distance and averaged over a constant area. With shrimp, readings are averaged across a number of abdominal segments, although the machines can also assess the color of other areas, such as the head or legs.
Color systems
There are a number of ways to describe absolute color. The Commission Internationale de l’Eclairage “Lab” system of color notation measures the absolute color of a sample on a three-dimensional scale of value, hue and chroma.
The color value or lightness has a scale of 0 for pure black to 100 for pure white. The hue has two components that distinguish opposing colors. The first is “a,” which represents the red-green scale, and the other is “b,” which represents the blue-yellow scale. Chroma or saturation indicates the amount of hue: positive a toward red, negative a toward green, positive b toward yellow and negative b toward blue.
The different models of color space notation have various advantages and disadvantages. However, if required, they can be interconverted from one to another using a series of mathematical calculations.
Background color response
In an experiment, Penaeus monodon shrimp were housed for six weeks in black or white tanks, and fed identical diets containing 70 mg/kg astaxanthin. Absolute color was then measured for raw and cooked animals from the black and white treatments. The color changes associated with cooking and the effects of background substrate color are defined in Fig. 1.

Animals from the black or white tanks could easily be distinguished when measured raw, and the differences between the two groups were accentuated once the animals were cooked. Animals from black tanks scored much higher on the red and yellow scales compared with animals from white tanks, which reflected their visible color. Despite this color difference, total levels of carotenoids were the same in the flesh of animals from the two groups.
Importantly, evidence from these color measurements indicated that the mechanism of color production was likely the same in animals exposed to either black or white tanks. It was simply the amount or amplitude of color that changed between the groups.
The final colors of the two treatments corresponded to subjective color scores of 24 and 31 on the Salmofan scale for the white and black treatments, respectively. This equated to scores of 5 and almost 11 on the Australian tiger prawn color chart, which has a scale ranging between 1 and 12, but the Salmofan is the internationally recognized color score standard.
Mechanisms of pigmentation
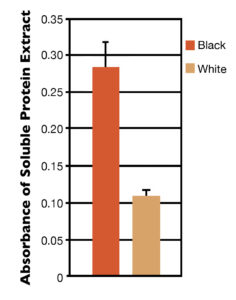
Using methods developed in this study, protein-soluble blue pigment was separated from the insoluble material in hypodermal tissues of animals exposed to either black or white substrates. There was a major difference in the amount of soluble blue pigment present in the shrimp tissues from the different treatments (Fig. 2). The blue protein was identified by western blotting as a carotenoid-binding protein known as crustacyanin.
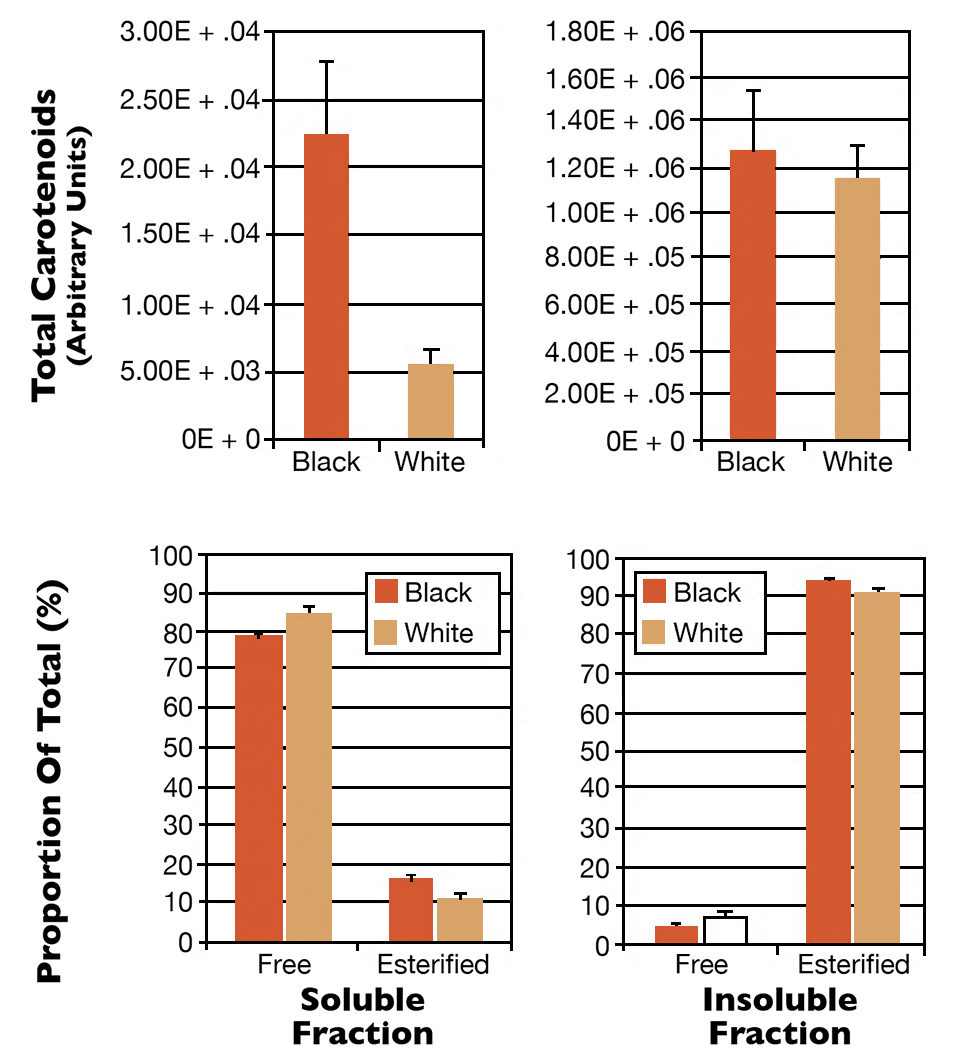
The soluble and insoluble fractions from this extraction were further analyzed for the amount and type of carotenoids they contained. The soluble fraction contained approximately 80 percent free astaxanthin, and the animals exposed to black substrates contained much more free astaxanthin than the animals exposed to white substrates (Fig. 3). The insoluble fraction contained over 90 percent astaxanthin esters, a more lipid-soluble form that may play a role in the storage of carotenoids in tissues.
The total amount of carotenoids present in the insoluble fraction was the same between the two treatments, and the majority of the carotenoid present in the tissue was found in the form of astaxanthin esters. These data indicated the observed color change was exclusively due to subtle changes in the amount of free astaxanthin in the soluble protein component of the hypodermal tissue, and these changes were unable to be detected unless the carotenoid types were separated and quantified.
Perspectives

Combined, these data clearly demonstrated a number of things. The blue protein came from the color protein crustacyanin binding to free astaxanthin, and there was less of this protein and less free astaxanthin in animals exposed to white substrates. The majority of astaxanthin present in hypodermal tissues was found in the form of astaxanthin esters, but this was not affected when animals were exposed to black or white substrates.
Most importantly, increases in the amounts of free astaxanthin in association with increased levels of crustacyanin had a profound effect on cooked shrimp color. This revealed the protein as a critical element in the deployment of carotenoids in shrimp tissues, that accumulation of this protein is triggered by exposure of animals to black substrates and that these changes in hypodermal proteins underlie improvements in overall shrimp color.
Editor’s note: This article was based on “Mechanisms of Colour Adaptation in the Prawn Penaeus monodon,” an article recently published in the Journal of Experimental Biology.
(Editor’s Note: This article was originally published in the January/February 2013 print edition of the Global Aquaculture Advocate.)
Authors
-
Nicholas M. Wade
CSIRO Food Futures Flagship
CSIRO Marine and Atmospheric Research
GPO Box 2583
Dutton Park, Queensland 4102 Australia -
Nigel P. Preston
CSIRO Food Futures Flagship
CSIRO Marine and Atmospheric Research
GPO Box 2583
Dutton Park, Queensland 4102 Australia -
Brett D. Glencross
CSIRO Food Futures Flagship
CSIRO Marine and Atmospheric Research
GPO Box 2583
Dutton Park, Queensland 4102 Australia
Tagged With
Related Posts

Health & Welfare
A holistic management approach to EMS
Early Mortality Syndrome has devastated farmed shrimp in Asia and Latin America. With better understanding of the pathogen and the development and improvement of novel strategies, shrimp farmers are now able to better manage the disease.

Aquafeeds
A look at protease enzymes in crustacean nutrition
Food digestion involves digestive enzymes to break down polymeric macromolecules and facilitate nutrient absorption. Enzyme supplementation in aquafeeds is a major alternative to improve feed quality and nutrient digestibility, gut health, compensate digestive enzymes when needed, and may also improve immune responses.
Innovation & Investment
A review of unit processes in RAS systems
Since un-ionized ammonia-nitrogen and nitrite-nitrogen are toxic to most finfish, controlling their concentrations in culture tanks is a primary objective in the design of recirculating aquaculture systems.

Health & Welfare
AHPN inferences based on behavior of vibrio bacteria
Vibrio parahaemolyticus, a strain of which is the cause of acute hepatopancreatic necrosis (AHPN), has both virulent and benign strains. This strain colonizes the stomachs of shrimp by the formation of a biofilm, which protects it from antibiotics and other potential treatments.


