Liming materials neutralize acidity, increase pH in bottom soil and water

Limestone is the most common raw material for making liming materials. Ordinary limestone is a relatively soft rock consisting of a mixture of calcium carbonate and a lesser amount of magnesium carbonate. Limestone containing only calcium carbonate is called calcite, while limestone with a calcium carbonate: magnesium carbonate ratio of exactly 1:1 is referred to as dolomite. These two minerals are fairly rare.
Limestone that is mostly calcium carbonate usually is called calcitic limestone, and limestone that has nearly equal proportions of calcium carbonate and magnesium carbonate often is considered dolomitic limestone by manufacturers of liming materials. Limestone may be classified based on its calcium and magnesium concentrations (Table 1).
Boyd, Calcium and magnesium concentrations, Table 1
| Type | Calcium (%) | Magnesium (%) |
|---|
Type | Calcium (%) | Magnesium (%) |
|---|---|---|
| Pure calcite | 40.0 | 0 |
| Calcitic limestone | 38.0-40.0 | < 1.2 |
| Pure dolomite | 21.7 | 13.2 |
| Dolomitic limestone | < 20.3 | > 12.0 |
| Ordinary limestone | Other compositions | Other compositions |
Agricultural limestone
Agricultural limestone is produced by finely pulverizing limestone in a rock crusher. This product also can be made from chalk and marl. Marl consists of (calcium and magnesium carbonates deposited in lakes that often are mixed with clay and seashells. Finely ground agricultural limestone dissolves more quickly and completely than coarsely ground limestone. Effects on total alkalinity concentrations in pond waters after applications of agricultural limestones with different particle fineness are illustrated in Fig. 1.
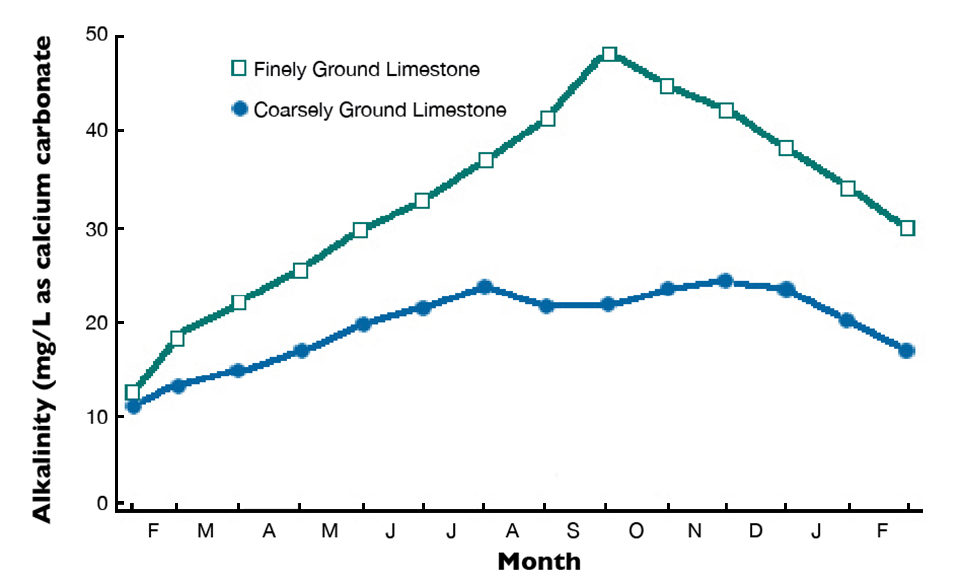
The fineness value of agricultural limestone is based on its particle size distribution. The solubilities of limestone particles of different sizes can be used to assign a characteristic efficiency value to each size class of particles. These efficiency values are used with the particle size distribution of individual samples to obtain the samples’ fineness values.
A sample that completely passes a No. 140 screen – with no particles larger than 106 µ in diameter – has a fineness value of 100 percent. Samples with coarser particles have lower fineness values. Agricultural limestone with a fineness value of 80 percent will dissolve only 80 percent as well as one with a fineness rating of 100 percent. Assuming two liming materials have equal abilities to neutralize acidity, it requires 1.25 kg of a material with a fineness rating of 80 percent to equal 1 kg of a material with a fineness rating of 100 percent.
Neutralizing values
The neutralizing value compares the acid-neutralizing capacity of a sample of liming material to that of a pure calcium carbonate standard. A product with a neutralizing value of 85 percent can neutralize 85 percent as much acid as an equal quantity of calcium carbonate that has a neutralizing value of 100 percent.
It is important to point out that dolomitic limestone has a maximum possible neutralizing value of 109 percent. However, there usually is no benefit in using dolomitic agricultural limestone – which often is more expensive than others – over ordinary agricultural limestone with neutralizing value near 100 percent.
Other liming materials
Limestone or one of the alternative sources of calcium and magnesium carbonates mentioned above can be burned in a kiln at high temperature to drive off carbon dioxide, leaving a residue of calcium and magnesium oxides called burnt lime. When treated with water, burnt lime reacts to yield calcium or magnesium hydroxides or hydrated lime.
Burnt and hydrated lime made from pure calcite have neutralizing values of 179 and 135 percent, respectively, while the neutralizing values would be 200 and 147 percent, respectively, for burnt lime and hydrated lime made from pure dolomite. However, lime usually is made from ordinary limestone or one of the alternative raw materials.
The raw material may not be completely burned, and the product can adsorb water during storage. Thus, the composition of burnt or hydrated lime often is not known exactly, and it is helpful to know the neutralizing value. It also is useful to know the fineness of the lime.
Application
Liming rates, traditionally expressed in terms of calcium carbonate, typically range 1,000 to 3,000 kg/ha. An agricultural limestone with a neutralizing value of 85 percent would have to be applied at 1,176 kg/ha to be equivalent to 1,000 kg/ha of calcium carbonate. The application rate also can be adjusted for fineness value. Suppose the product with 85 percent neutralizing value has a fineness value of 80 percent. The application rate adjusted for both neutralizing and fineness values would be 1,470 kg/ha.
Because of its higher neutralizing value, lime is used at lower application rates than agricultural limestone. Nevertheless, even at low application rates, lime can raise water pH to 12 or more. Agricultural limestone, on the other hand, does not cause pH to rise above about 8.5.
In ponds containing fish or shrimp, lime should not be applied at more than 50 kg/ha to avoid excessively high pH. The greatest use of lime is for disinfection of bottom soils in empty ponds between crops by applying 1,000 kg/ha or more in a single treatment to raise pH to 12 or above and kill unwanted organisms.
In many instances, information on neutralizing and fineness values of liming products is not available. A common practice in traditional agriculture is to apply 50 percent more liming material than the recommended lime requirement in order to compensate for low neutralizing value, fineness value or both. This approach also can be used in aquaculture.
All types of liming materials react similarly to neutralize acidity and increase pH in bottom soil and water. They also react with carbon dioxide to form bicarbonate and release calcium and magnesium, increasing both total alkalinity and total hardness concentrations in water.
Liming materials are at best sparingly soluble, and they should be spread as uniformly as possible over pond bottoms between crops or spread over the entire water surface of ponds. Liming materials also react to bind phosphorus when initially applied to ponds. Thus, fertilizers should not be applied for about a week following liming.
(Editor’s Note: This article was originally published in the July/August 2011 print edition of the Global Aquaculture Advocate.)
Author
-

Claude E. Boyd, Ph.D.
Department of Fisheries and Allied Aquacultures
Auburn University
Auburn, Alabama 36849 USA
boydce1@auburn.edu
Tagged With
Related Posts

Responsibility
Drying, liming, other treatments disinfect pond bottoms
The traditional way of destroying organisms in pond bottoms is thorough dry-out for a week or longer. Fish toxicants and liming can kill unwanted parasites.

Responsibility
A look at various intensive shrimp farming systems in Asia
The impact of diseases led some Asian shrimp farming countries to develop biofloc and recirculation aquaculture system (RAS) production technologies. Treating incoming water for culture operations and wastewater treatment are biosecurity measures for disease prevention and control.

Responsibility
Calcium and magnesium use in aquaculture
Aquatic plants and animals get the essential nutrients calcium and magnesium from water and food. Calcium concentrations impact the hydration and development of eggs in a hatchery, where calcium carbonate precipitation can be troublesome.

Responsibility
Constantly changing pH unavoidable, completely normal
Prof. Claude Boyd discusses the importance of pH for farmed fish and shellfish, the normal and natural fluctuations and how aquaculture systems can manage it.

