Safe concentration of a toxicant is called the maximum allowable toxicant concentration
Aquaculturists sometimes are confronted with the toxicity of natural or man-made substances to fish, shrimp, or other culture species. Naturally occurring toxicity can result from low dissolved-oxygen concentrations and high concentrations of ammonia, carbon dioxide, nitrite, or hydrogen sulfide. Toxicity also can result from contamination of culture systems by pesticides, heavy metals, or industrial chemicals.
Lethal concentration
The potential toxicity of chemical substances often is presented as their LC50. LC50 is the concentration of a substance that is lethal to 50 percent of the organisms in a toxicity test. LC50 can be determined for any exposure time, but the most common exposure period is 96 hours. Other common durations are 24, 48, and 72 hours.
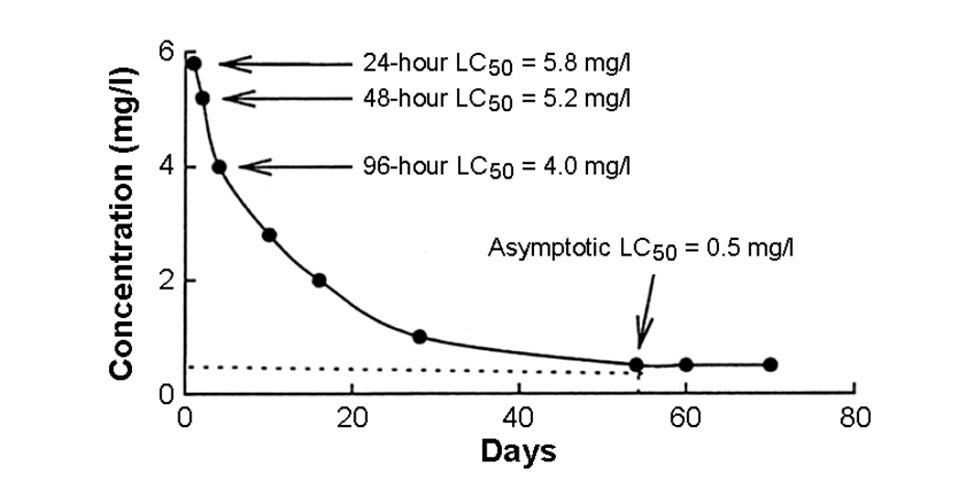
As a general rule, the longer the exposure, the lower the LC50. If the exposure is long enough, an asymptotic LC50 value can be obtained (Fig. 1). The asymptotic LC50 is not time-dependent.
Toxicity testing
Toxicity tests usually are conducted in glass containers that hold 1 liter or more of toxicant-treated water and several individuals of the test species. Typical tests use a range of toxicant concentrations and a control with no toxicant.
In static tests, water exchange is not used and wastes may accumulate. Moreover, the concentration of the toxicant can decline through absorption by the test animals, or chemical or biological degradation. Thus, flow-through tests (Fig. 2) in which the treated water is continuously renewed to rid the system of wastes and maintain a constant toxicant concentration are more reliable.
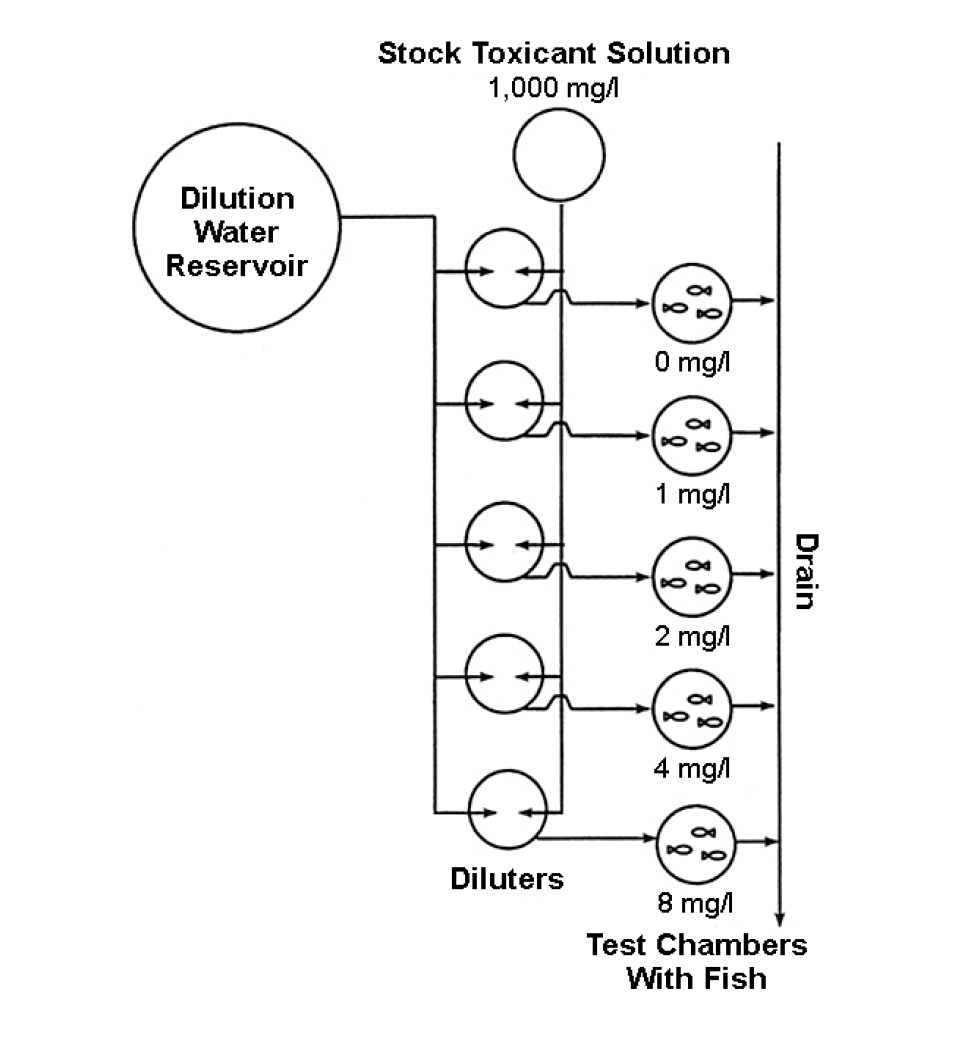
Specific guidelines for conducting toxicity tests include procedures for the acclimation of test organisms and limits on organism size and weight per volume of water, temperature range, light intensity, and other factors. Mortality must be checked frequently with dead test organisms removed promptly. Standard Methods for the Examination of Water and Wastewater, published by the American Public Health Association, is an excellent reference on toxicity testing.
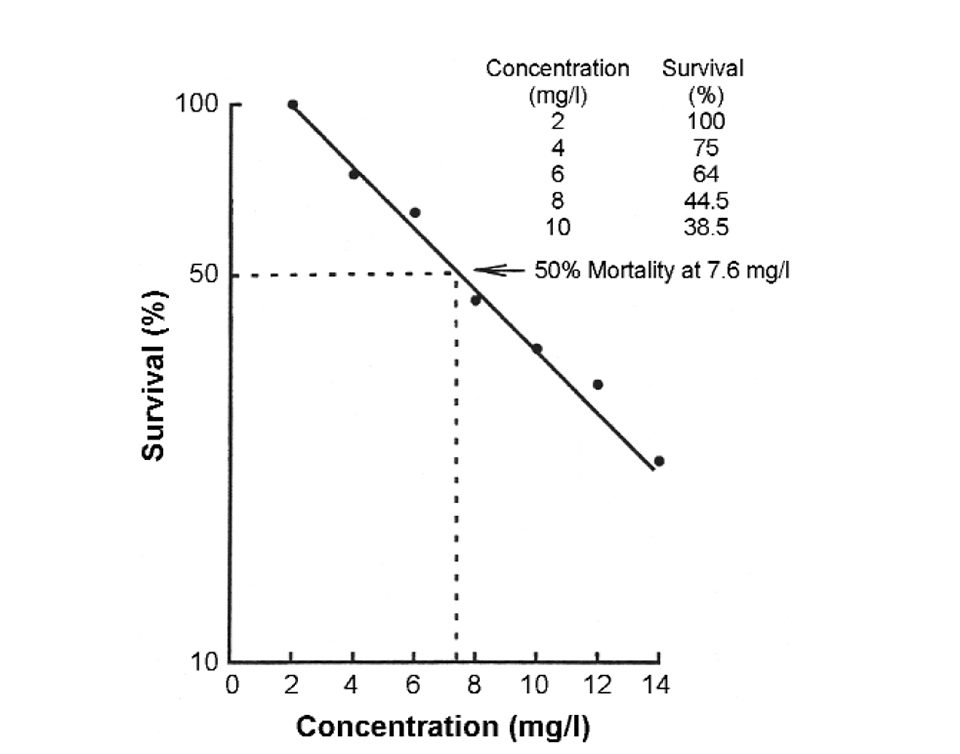
When tests terminate, the mortality for each toxicant concentration is adjusted for mortality in the control. The mortality percentage is plotted on semi-log paper versus the toxicant concentration, and the concentration that killed 50 percent of the test organisms is calculated graphically or by statistical techniques (Fig. 3).
Variability
There can be great variation in LC50 among toxicity tests conducted in different laboratories. This results from differences in water quality in the test containers, temperature, species and source of test organisms, and other factors.
For example, the toxicity of most substances increases with increasing temperature. Water quality variables such as pH and total alkalinity also can have large effects on toxicity. Copper and most other metals are much less toxic at total alkalinities of 100 milligrams per liter or greater than at lower alkalinities. The concentration of total ammonia nitrogen necessary to kill 50 percent of test organisms is greater at a pH of 7 or below than at higher pH.
Aquaculture uses
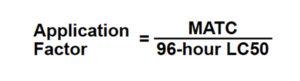
In evaluating the cause of mortality in aquaculture animals, one may find in scientific literature a range of LC50 values for a particular toxicant. The LC50 value most appropriate for reference is the one obtained in a toxicity test in which conditions were similar to those of the aquaculture system at the time of mortality.
In aquaculture, the concentration of potential toxicants should be below that which causes mortality or adverse effects on growth and reproduction. This information is directly available from toxicity tests with a duration equal to the entire life cycle of the test species.
The safe concentration of a toxicant is called the maximum allowable toxicant concentration or MATC. There is much less life cycle toxicity data available than 96-hour LC50 data. However, it is possible to relate a 96-hour LC50 to the MATC by an application factor by applying the formula in Fig. 4.
Application factors typically range 0.01-0.1, but for some toxicants, the factor may be 0.001. Application factors tend to be smaller for highly toxic compounds than less-toxic ones. In aquaculture, an application factor of 0.05 is suitable for most natural toxins, while a factor of 0.01 should be used for industrial chemicals or pesticides.
Factor example
To illustrate the use of application factors, suppose the 96-hour LC50 to the culture species for unionized ammonia (NH3) is 1.2 milligrams per liter. The MATC would be 1.2 milligrams per liter x 0.05 = 0.06 milligrams per liter. Thus, if the NH3 concentration remains below 0.06 milligrams per liter, the culture species should suffer no adverse effects.
Of course, toxicity might occur at concentrations of NH3 below 1.2 milligrams per liter, for that value represents the concentration necessary to kill half of the test organisms. A much lower concentration could be expected to cause incipient mortality (Fig. 3).
Sometimes it is not possible to find LC50 data for a desired temperature. Toxicity usually doubles with a 10 degrees C increase in temperature. Thus, if 2 milligrams per liter is the 96-hour LC50 of a substance at 20 degrees-C, the corresponding values at 25 and 30 degrees-C could be estimated as 1.5 milligrams per liter and 1 milligrams per liter, respectively.
(Editor’s Note: This article was originally published in the February 2005 print edition of the Global Aquaculture Advocate.)
Now that you've reached the end of the article ...
… please consider supporting GSA’s mission to advance responsible seafood practices through education, advocacy and third-party assurances. The Advocate aims to document the evolution of responsible seafood practices and share the expansive knowledge of our vast network of contributors.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year.
Not a GSA member? Join us.
Author
-

Claude E. Boyd, Ph.D.
Department of Fisheries and Allied Aquacultures
Auburn University
Alabama 36849 USA
Tagged With
Related Posts

Health & Welfare
Ammonia toxicity degrades animal health, growth
Ammonia nitrogen occurs in aquaculture systems as a waste product of protein metabolism by aquatic animals and degradation of organic matter, or in nitrogen fertilizers. Exposure can reduce growth and increase susceptibility to diseases in aquatic species.
Innovation & Investment
A review of unit processes in RAS systems
Since un-ionized ammonia-nitrogen and nitrite-nitrogen are toxic to most finfish, controlling their concentrations in culture tanks is a primary objective in the design of recirculating aquaculture systems.

Health & Welfare
American fisheries society calls for immediate-release fish sedatives
The absence of practical immediate-release sedatives for fish jeopardizes fish, fisheries, fish culture and research, posing risks to aquatic resources as well as those handling fish.

Responsibility
Ammonia nitrogen dynamics in aquaculture
The major sources of ammonia in aquaculture ponds are fertilizers and feeds, and problems with high ammonia are most common in feed-based aquaculture.

