Las Bolitas Syndrome has a severe impact on the digestive system of P. vannamei larvae, contrasting with the absence of pathogenic features in healthy larvae

During the late 1980s, 1990s and early 2000s, the most predominant pathologies in shrimp hatcheries in Latin America were Larval Bolitas Syndrome (LBS), Zoea 2 Syndrome, and Mysis Molt Syndrome. During the same period, the main pathologies reported in post-larvae (PL) were luminescent bacteria. After 2015, disease outbreaks with high rates of mortality were more commonly seen in post-larval production, e.g., post-larval Acute Hepatopancreatic Necrosis Disease, AHPND (PL-AHPND), Translucent Post-Larva Disease (TPD), and others.
Larval Bolitas Syndrome (LBS) is a condition initially characterized by a distinctive pathology of the hepatopancreas, where there is a cellular sloughing of the epithelium of the hepatopancreas, forming spheres, which eventually move into the upper gut. Gross pathology of LBS normally develops in a matter of hours, from healthy well-fed zoea to empty moribund animals. At the same time, the larvae become bioluminescent, which is accompanied by changes in behavior and a loss of appetite. Although LBS may occur during post-larval (PL) stages, massive mortalities, reaching up to 90 percent, have been observed primarily in the earlier zoea and mysis stages. A distinctive characteristic of LBS is the presence of bolitas (“small balls” or “spheres” in Spanish) in the hepatopancreas, which eventually migrate to the intestine.
LBS has been associated with infection of Vibrio spp. Zoea 2 syndrome was only associated with V. harveyi and V. alginolyticus, and the pathogenic species associated with mysis syndrome has never been identified. The nocturnal luminescence seen in hatcheries in both Asia and the Americas was typically attributed to luminescent Vibrio. In more recent times, outbreaks of sudden and acute mortalities in penaeid shrimp hatcheries typically start in the PL stages, from an active and apparently healthy state to moribund and dead. The speed and virulence at which these massive mortalities occur have been observed in many production facilities, and in most cases have been associated with, or linked to, different strains of Vibrio.
Intriago et al. provided evidence that the cause of these rapid mortality events was a species of Vibrio carrying the same plasmids as the VpAHPND reported as causing acute hepatopancreatic necrosis disease (AHPND) in pond-cultured shrimp elsewhere. The condition was tentatively named post-larvae AHPND (PL-AHPND) to differentiate it from other pathologies affecting penaeid shrimp larvae. In Asia, Translucent Post-Larva Disease (TPD) has been the main cause of larval disease and mortality, where the causative agent is a strain of V. parahaemolyticus carrying a particular gene. In India, a similar condition referred to as zoea 2 syndrome has been described affecting zoea 2 of P. vannamei.
In September 2023, some shrimp larval production facilities in the Latin America region experienced high mortalities in the zoea stages 2 and 3. Given the high mortality rate, these tanks were discarded. The causal agent associated with those mortalities has not been reported. Microscopic observation of the affected larvae revealed the presence of “bolitas” (spheres) in the hepatopancreas. Generally, the clinical indicators and the macroscopic appearance on wet mounts aligned with what was previously identified as LBS.
This article – summarized from the original publication (Intriago, P. et al. 2024. Las Bolitas Syndrome in Penaeus vannamei Hatcheries in Latin America. Microorganisms 2024, 12(6), 1186) – study reports the microbiological, polymerase chain reaction (PCR) tests and histological findings of healthy and of diseased animals suffering from this this condition.
Study setup
Samples of P. vannamei larvae from two hatcheries in Latin America were sampled (precise location details are withheld acknowledging the facilities request for confidentiality). Samples for microbiology, PCR and histopathology were taken from two tanks from each hatchery. Hatcheries were selected because one of the hatcheries had reported heavy mortality at zoea stages 2–3, while the other hatchery, with apparently healthy zoea 2–3, was selected as the control. It should be noted that shrimp sampled for PCR and histology were different individuals from the same populations. To protect client privacy, the country and the precise location of each culture facility from which the samples were obtained will not be disclosed.
For detailed information on the collection, processing and analyses of larval shrimp carried out in this study, refer to the original publication.
The OIE should delist IHHNV as a reportable farmed shrimp pathogen
Results and discussion
This study describes the histopathological lesions, PCR and microbiology of samples of P. vannamei zoea collected from two different hatcheries in Latin America: one with an outbreak of Larval Bolitas Syndrome, LBS, and the other with apparently healthy animals. Of all the pathogens that were analyzed by PCR, the only key differences between the LBS and healthy zoea were the detection of Vibrio and more abundant Rickettsia-like bacteria, RLB, in the diseased zoea.
The presence of the Wenzhou shrimp virus – WzSV8 – in the healthy zoea requires more study; this RNA virus has been found in a wide range of different environments and regions including wild shrimp broodstock, and its effect in penaeid production needs to be the subject of further studies.

Microbiology showed that the total number of culturable bacteria (TSA) was one order of magnitude higher in the affected zoea and the number of presumptive Vibrio was almost two orders of magnitude higher when compared to the healthy zoea. Presumptive Vibrio represented 17 percent and 6 percent of the total bacterial population in the unhealthy and healthy zoea, respectively. Green colonies (on TCBS) and mauve colonies which are presumptive V. parahaemolyticus represented 0.2 percent and 82 percent of the total Vibrio count in affected zoea, while a reverse pattern was seen in the healthy zoea which had 56 percent and 2 percent, respectively.
This finding is interesting because the common assumption is that green colonies on TCBS and purple colonies on CHROMagar™ Vibrio (a selective medium for isolation and detection of particular Vibrio species) typically represent V. parahaemolyticus. In this regard, researchers studying the phenotypic characteristics and growth kinetics of three V. parahaemolyticus strains with different virulence and one non-pathogenic strain found that independent of the virulence of the strain, high metabolic diversity was present, which yielded different-colored phenotypes on the CHROMagar™ Vibrio.
It is important to note that all 14 strains were identified by PCR as members of the genus Vibrio, so it can be concluded that API 20E (test kit to identify gram negative bacteria) results (21 percent false identification at a genus level) were not reliable and should be taken with caution. While other researchers have reported that the API 20E is a valid system for use in the identification of the more commonly occurring members of the family Vibrionaceae, the system has been reported to result in false negatives. API identification is based on biochemical profiles, but it has been found that biochemical profiles and genotype are not necessarily associated with virulence potential. The relationship between the variation or differences in the API 20E identifications and the possible virulence and genus variation of each strain requires further research.
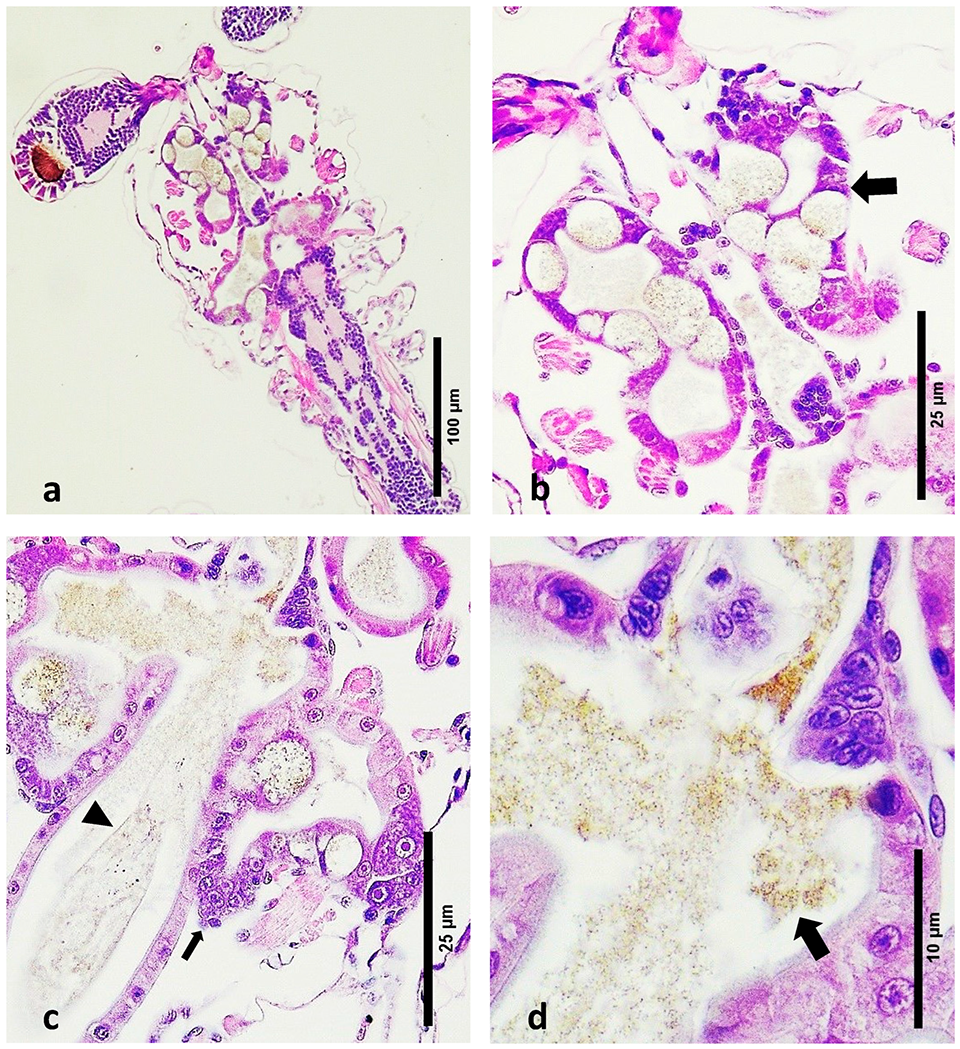
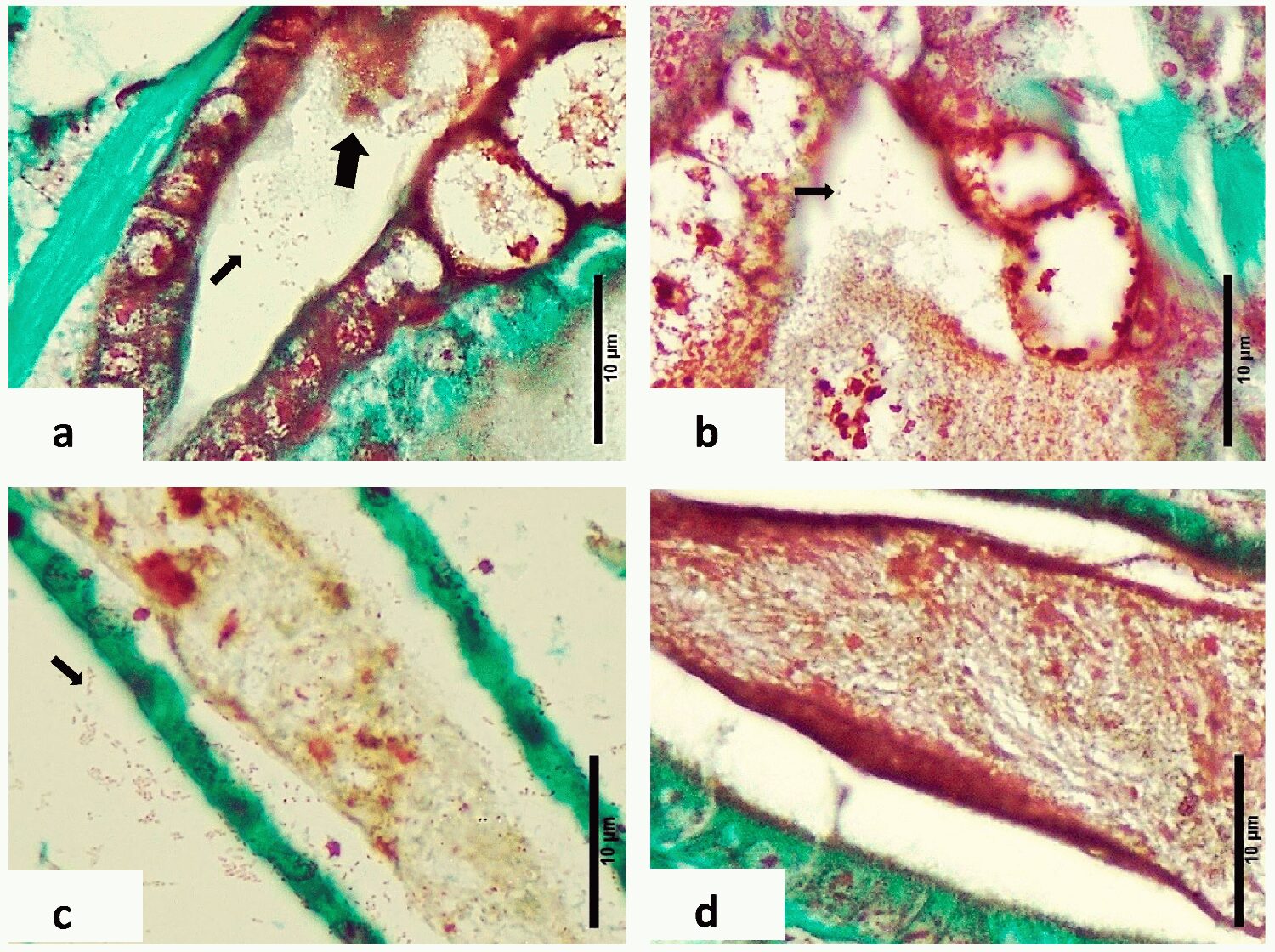
A comparison between the histology in this study and the first report of LBS reported by Morales (Memorias del Primer Congreso Ecuatoriano de Acuicultura; Escuela Superior Politécnica del Litoral: Guayaquil, Ecuador, 1992; pp. 203–207) is not possible. Unfortunately, only transmission electron microscopy (TEM) images were presented, and no tissue sections stained with H&E were recorded. The publication by Robertson et al. shows the presence of melanized necrotic bundles in the hepatopancreatic tubules, but we did not observe this in the present research; the most likely reason was that the animals they used for the histology were not zoea but were least at the PL1 stage.
An important difference between the pathology presented here for LBS and for PL-AHPND is that there is no massive sloughing of cells in the hepatopancreas, as was described for PL-AHPND. In addition, PL-AHPND was never found or described in zoea. In addition, no AHPND PirAB genes were detected, nor were other pathogenic genes. A similar material present in shrimp larvae reared in China and suffering mortalities during zoea stage 2 has been described by other authors; in their case this was associated with a strain of V. alginolyticus.

Historically, the Latin American shrimp sector has followed the Ecuadorian model, where post-larvae are produced from broodstock obtained from production ponds. Broodstock are selected based on weight and then transferred to a hatchery to produce the next generation of post-larvae. This process provides little to no long-term control over biosecurity. In addition, high concentrations of Vibrio spp. can be very common in non-biosecure penaeid hatcheries or those that are not specific pathogen-free, both free and as attached to the larvae.
From hatching through to harvest, the microbiological environment is a soup of bacteria and virus-like particles (VLPs). As the larvae transition from a diet based on algae as zoea to animals that source proteins as mysis, the larvae then undergo a dramatic change in the volume of the hepatopancreas and the biochemistry of the digestive enzymes. In zoea, the filtration of particles is almost indiscriminate. As the zoea stages are exposed to very high concentrations of bacteria, free-living and attached – including Vibrio that are easily ingested and able to pass into the digestive tract – the resulting detrimental effects can be observed contingent on several factors such as the larval sub-stage involved, the Vibrio species present, and their concentration.
In 1997, Intriago and Jimenez (unpublished report) replicated the Bolitas syndrome in P. vannamei zoea using a luminescent strain of V. harveyi at concentrations as low as 10^3 cell per mL. Interestingly, this strain was isolated from diseased farm-cultured P. vannamei affected by haemocytic enteritis. They postulated that pathogens could be bouncing back from hatcheries to the broodstock or vice versa and that the differences found in the histopathology between the larvae and adults could have been attributed to the differences in the degree of organ development, or to the pathogen species, its virulence and its concentration.
The key event in the appearance of diseases could be the presence of stress (due to temperature, salinity, density, toxins, and others) because of fluctuations in the environment, and this exerts a change in the host-pathogen interaction and the transmission of bacteria between species. Such modifications act on pathogens to facilitate their increased transmission between individual hosts and increased contact with new host populations or species and on the selection, pressure leading to the dominance of pathogen strains adapted to these new environmental conditions.
Unfortunately, it is not possible to compare the histology of this study with that of previous published reports, although the histology of this study resembles the zoea 2 syndrome reported by Kumar et al. However, the differences in pathology could be the product of different responses by the host to a wide dynamic bacteria genotype and the concentration of bacterial pathogens. In addition, two pathogens could also produce the same macroscopic pathology but the differences in the damage at the tissue level will depend on all the factors described above.
Perspectives
From the information presented here, it is clear that no virus was involved in this disease outbreak. Although some viruses were detected by PCR, no inclusion bodies or associated lesions were found. We can also eliminate common Vibrio pathologies such as AHPND or the new highly lethal V. parahaemolyticus described for P. vannamei larvae carrying the genes Vhp1- and Vhp-2. All 14 bacterial strains isolated were identified as Vibrio by PCR.
It is tempting to suggest that Vibrio played a role in the pathogenicity, however, we cannot rule out RLB or some toxicity in the culture water. We can, however, conclude that Vibrio species as pathogenic or as opportunistic bacteria play an important role in both LBS and healthy tanks, and in general they were characterized by high metabolic diversity which yielded different-colored phenotypes on the CHROMagar™ Vibrio.
The histopathological examination of LBS-affected zoea stages 2–3 larvae revealed significant pathological changes, including epithelial cell detachment, hepatopancreatic necrosis, disrupted peritrophic membranes and the presence of Gram-negative bacteria. These findings highlight the severe impact of LBS on the digestive system of P. vannamei larvae, contrasting starkly with the intact structures and absence of pathogenic features in healthy larvae. Further research is needed to fully understand the etiology of LBS and develop effective interventions to mitigate its effects in shrimp hatcheries.
Samples were sent by clients for monitoring shrimp health status or disease outbreaks in their hatcheries. Animals (zoea) used as controls were generally healthy and were collected as reference specimens to compare with the affected animals. This study was not an epidemiological study but a simple prevalence analysis of various shrimp pathogens in randomly provided samples from a hatchery that reported mortality. Unfortunately, owners and managers do not disclose information regarding the protocols used or the physical and chemical parameters of the culture, as they believe it could damage the hatchery’s reputation. Consequently, we are unable to conduct more extensive sampling or provide detailed information related to the larval culture.
Now that you've reached the end of the article ...
… please consider supporting GSA’s mission to advance responsible seafood practices through education, advocacy and third-party assurances. The Advocate aims to document the evolution of responsible seafood practices and share the expansive knowledge of our vast network of contributors.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year.
Not a GSA member? Join us.
Author
-
Pablo Intriago, Ph.D.
Corresponding author
South Florida Farming Corporation, 13811 Old Sheridan St, Southwest Ranches, FL 33330, USA
Tagged With
Related Posts

Health & Welfare
Determination of the infectious agent of Translucent Postlarva Disease in Pacific white shrimp
Systematic analyses confirm novel V. parahaemolyticus is causative agent associated with Translucent Postlarva Disease, which hit China in 2020.

Health & Welfare
Efficacy of natural products and antibiotics in shrimp hatcheries
This study evaluated through in vitro analyses the antimicrobial effectiveness of natural products used for P. vannamei bacterial diseases and antibiotics against pathogenic Vibrio strains circulating in Ecuadorian hatcheries.

Health & Welfare
Asepsis key to prevent contamination in shrimp hatcheries
Maintaining biosecurity and asepsis in larval shrimp production is a key component of the production chain in Ecuador, which requires the production of 5.5 billion larvae monthly from 300-plus hatcheries.

Health & Welfare
How good are your shrimp postlarvae?
Stocking the best quality shrimp postlarvae, healthy and free of pathogens, is a critical management step with significant effects on the production and profitability of a shrimp farm.



