Constant darkness resulted in more obscure body color and altered the hepatopancreas metabolism and intestinal microbiota

Most species have developed internally driven circadian rhythms [natural, internal processes that regulate the sleep-wake cycle and repeat roughly every 24 hours] in their physiology and behavior that are attuned to changes in the daily 12 hours light:12 hours dark cycle. The photoperiod profoundly influences the circadian rhythm of biochemical, physiological and behavioral processes in almost all living organisms.
Extensive research has shown that changes in the light-dark cycles are closely associated with various metabolic disorders. In aquatic animals, the cycle acts as an important biological factor that influences the entire life cycle from embryonic development to sexual maturation.
In recent years, growing evidence has shown that the effects of the light-dark cycle on various aquatic species are diverse: some species have a natural preference for dark environments while others have an improved physiological state under high light intensity.
Different light-dark cycles could affect growth, digestibility and physiological metabolism in fish. In crustaceans, the hepatopancreas is an important organ and the main site for nutrient digestion, absorption, and metabolism. The Pacific white shrimp (Litopenaeus vannamei) is the main species of shrimp cultured worldwide. Until now, evidence regarding the regulation of physiological metabolic process under different light-dark cycles is limited in L. vannamei. The hepatopancreas and intestine can be the potential targets for studying the responsive mechanism of shrimp in response to different light-dark cycles.
This article – adapted from the original publication (Jiao, L. et al. 2021. Influence of Light/Dark Cycles on Body Color, Hepatopancreas Metabolism, and Intestinal Microbiota Homeostasis in Litopenaeus vannamei. Front. Mar. Sci., 29 November 2021) – investigated the effects of different light/dark cycles (12 h light/12 h dark, 0 h light/24 h dark) on the hepatopancreas metabolism and intestinal microbiota in L. vannamei.
Study design
This research was carried out at the Laboratory of Fish Nutrition, School of Marine Sciences, Ningbo University, in Ningbo, China. A total of 180 L. vannamei juveniles (individual weights ~0.72 grams) were randomly divided into two groups: a natural light treatment group (12 hours light:12 hours dark) and a dark treatment group (0 hours light:24 hours dark), with three replicates per group.
After an eight-week feeding trial, the shrimp body color was captured using digital cameras and then analyzed with a commercial software program. Various samples of shrimp hemolymph, hepatopancreas, and intestinal contents were collected and analyzed.
For detailed information on the experimental design and animal husbandry; and analytical testing and analyses of the samples collected, refer to the original publication.
Results and discussion
The change in body color in crustaceans has received much attention as a conspicuous and quantifiable phenomenon related to various physiological and ecological factors. Crustaceans have the ability to change coloration in response to photoperiod, which may play roles including photoprotection and enhancing camouflage in unique marine environments.
In our study, one interesting finding was that the body color of L. vannamei became darker after dark treatment in an eight-week feeding trial, which may be related to the decreased expression of specific genes. Although the molecular mechanism of crustacean body color change is not clear, several studies have indicated that the regulation of body color can be associated with the expression of a gene for the pigment crustacyanin.
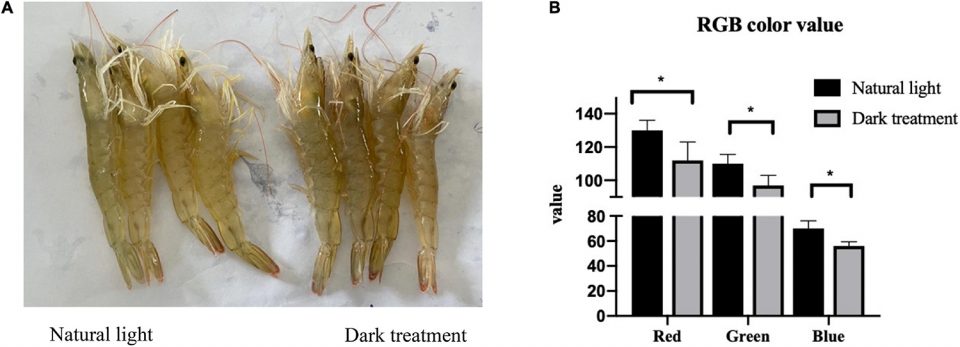
Astaxanthin, a carotenoid [yellow, orange and red organic pigments produced by plants, algae, several bacteria and fungi] pigment found in nature, appears to be the main pigment responsible for color in crustaceans, accounting for approximately 65 to 98 percent of all the carotenoids found in shrimp species. The stability of the highly reactive astaxanthin pigment results from interactions with forms of the pigment crustacyanin.
Researchers have reported that L. vannamei infected with various Vibrio spp. consumed less feed and their body color tended to be darker. Our intestinal microbiota analysis confirmed that the relative number of Vibrio increased after the dark treatment. Consequently, we believe that the dark treatment in our experiment decreased the gene expression of crustacyanin and increased intestinal Vibrio numbers, and resulted in the changed body color in L. vannamei. However, the molecular mechanism involved remains unclear.
Most organisms have evolved an internal circadian clock that drives circadian rhythms in their metabolism, physiology, and behavior. The circadian clocks use a 24-hour light-dark cycle as the environmental signal to establish internal (endogenous) circadian timing systems that synchronize several biological functions. In our study, we observed that certain circadian clock genes were downregulated [their expression was decreased] under constant darkness treatment in the hepatopancreas of L. vannamei, including some involved in insulin regulation.
In invertebrates, the role of the insulin pathways includes not only glucose steady state (homeostasis) but also the regulation of a variety of fundamental processes such as growth, aging, and reproduction. Overall, our results provide the first evidence that constant dark treatment could influence hormone regulation in the hepatopancreas of L. vannamei.
Green-lighting growth: Green LED light shows promise in flounder farming
The involvement of the light-dark cycle as an important regulator of immune functions has been extensively described in mammals, but there is a paucity of information on the influence of this biological phenomenon in aquatic animals. Limited studies have reported that the innate immune system had a circadian rhythm based on the light-dark cycle in various fish species.
We also found that the dark treatment significantly suppressed the expression of immune-related genes in the hepatopancreas of L. vannamei, including some involved in the activation of the shrimp immune defenses against invasive pathogens. These results indicated that constant dark treatment impaired the immune function in the hepatopancreas of L. vannamei.
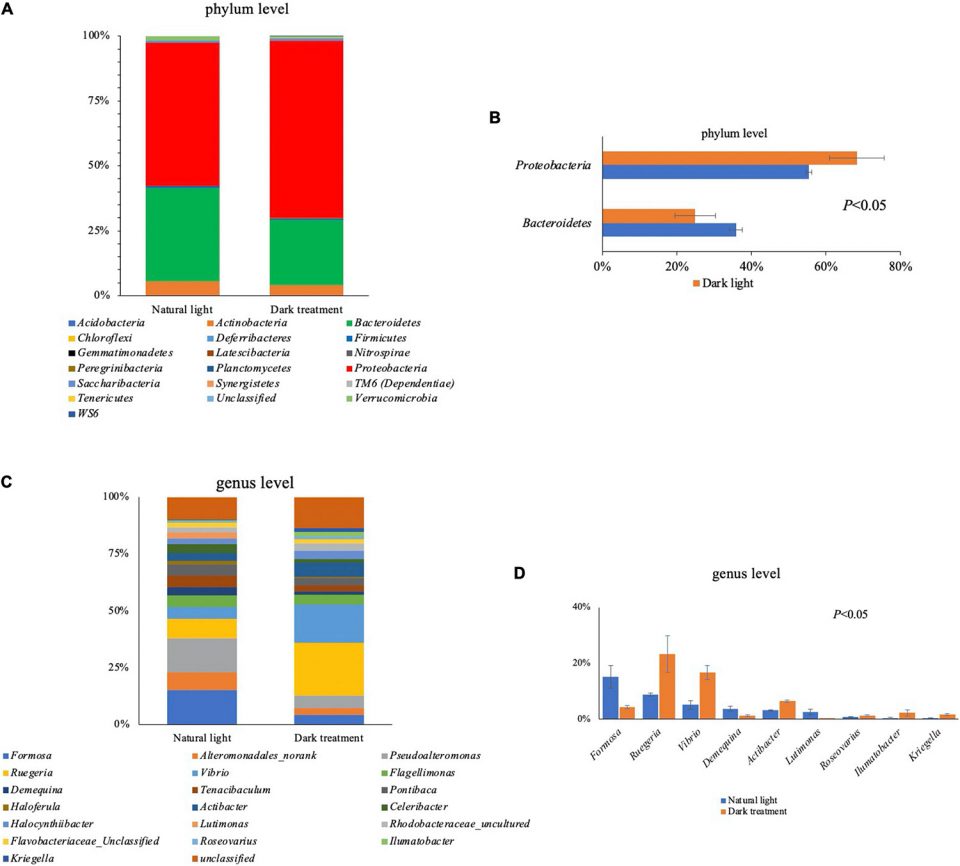
The intestinal microbiota modulates host physiological processes and plays a vital role in promoting and maintaining the health of the host. Our data showed that the dark treatment significantly increased the relative abundance of several bacterial genera, including Ruegeria, Vibrio, Actibacter, Roseovarius, Ilumatobacter and Kriegella in the intestines of L. vannamei. It is relevant to note that the dark treatment promoted the proliferation of pathogenic bacteria like Vibrio spp., the most typical and well-known pathogen causing vibriosis infections in aquatic animals.
Perspectives
Overall, our findings indicated that constant darkness resulted in more obscure body color, altered hepatopancreas metabolism, and intestinal microbiota homeostasis in L. vannamei. Genes involved in regulating nutrition metabolism, body-color formation, diurnal rhythm, immune function, hormone levels, and other functions were downregulated after constant darkness for eight weeks. Further intestinal microbiota analysis showed that dark treatment-induced alterations in intestinal bacterial abundances and circadian rhythms increased susceptibility to various pathogens, and decreased nutrition metabolism.
Our results provide an important reference for further understanding of the impact of different light-dark cycles on shrimp physiological processes (including body color, hepatopancreas metabolism, and intestinal microbiota), which could help improve shrimp producers by adjusting light-dark cycles in controlled shrimp farming systems.
Now that you've reached the end of the article ...
… please consider supporting GSA’s mission to advance responsible seafood practices through education, advocacy and third-party assurances. The Advocate aims to document the evolution of responsible seafood practices and share the expansive knowledge of our vast network of contributors.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year.
Not a GSA member? Join us.
Authors
-
Dr. Lefei Jiao
Laboratory of Fish Nutrition, School of Marine Sciences, Ningbo University, Ningbo, China
-
Dr. Tianmeng Dai
Laboratory of Fish Nutrition, School of Marine Sciences, Ningbo University, Ningbo, China
-
Dr. Xinyue Tao
Laboratory of Fish Nutrition, School of Marine Sciences, Ningbo University, Ningbo, China
-
Dr. Jingjing Lu
Laboratory of Fish Nutrition, School of Marine Sciences, Ningbo University, Ningbo, China
-
Dr. Qicun Zhou
Corresponding author
Laboratory of Fish Nutrition, School of Marine Sciences, Ningbo University, Ningbo, China
Tagged With
Related Posts

Intelligence
Evaluating effects of light, feeding, circadian clock in turbot
Function and regulation of the turbot's circadian timing system are assessed at the molecular and behavioral levels to optimize welfare and production.

Health & Welfare
LED lighting technology provides unique benefits for aquaculture
Environmental lighting, which synchronizes all stages of aquatic life, is an important consideration as more aquatic animals are produced indoors in highly controlled environments. Artificial lighting should deliver uniform light across the water’s surface in rearing tanks and mimic not only photoperiod, but also light color and intensity.

Health & Welfare
Tilapia fry perform similarly under varied photoperiods
The growth and survival of red tilapia fry were not significantly affected by different photoperiods in three 30-day experiments. Results suggested that it is not necessary to maintain constant lighting or a constant light/dark circadian scheme.

Health & Welfare
Evaluating passive acoustic telemetry to monitor gilthead sea bream juveniles
An evaluation of passive acoustic telemetry techniques as a tool to monitor welfare of gilthead sea bream juveniles implanted with acoustic transmitters.



