Goals include estimates of heritability, phenotypic and genetic variation and correlation, genotype-environment interaction
Most shrimp cultured worldwide are either collected from the wild or are offspring from wild-caught broodstock. This practice is risky because wild-caught shrimp may be carriers of pathogens, including viruses. Several of these viruses have devastated the global shrimp farming industry in recent years, resulting in the emergence of novel production systems that rely on pathogen exclusion. Additionally, there are concerns about the ecological effects of harvesting wild shrimp for aquaculture, and this activity has been implicated in changing the dominant species composition of wild shrimp caught by fishermen in coastal Ecuador.
A significant disadvantage of culturing wild-caught shrimp is the inability of the farmer to benefit from domestication and genetic improvement. In the U.S. poultry industry, selective breeding has resulted in dramatic improvements in growth rate, feed conversion efficiency and reproductive performance. For example, the chickens that we eat today grow twice as fast on half the amount of feed as the chickens of 50 years ago.
This improvement is due, in large part, to the selective breeding practices of poultry breeders. Unfortunately, benefits accrued from the selective breeding of shrimp lag far behind those realized by the poultry industry. However, many penaeid shrimp possess characteristics amenable to selective breeding, including the ability to close the life cycle in captivity, a short generation time, and high fecundity.
During the past decade there has been an emergence of shrimp breeding programs in Asia and the Americas, and the Oceanic Institute (OI) has played a significant role in establishing some of the fundamental principles of operating such a program. In this article, we highlight OI’s selective breeding program for the Pacific white shrimp (Litopenaeus vannamei) and summarize major findings.
Critical to the success of the breeding program at OI is the use of specific pathogen free (SPF) shrimp stocks. The SPF concept was developed by terrestrial animal breeders and refers to the use of animals that are free of specifically listed pathogens. In 1989, the U.S. Marine Shrimp Farming Program (USMSFP), funded by the U.S. Department of Agriculture (USDA), developed the first population of SPF L. vannamei at OI for distribution to U.S. shrimp farmers. SPF populations were established following the guidelines of the International Council for the Exploration of the Seas (ICES). These guidelines stipulate that only disease-causing organisms that can be reliably diagnosed and physically excluded from a facility can be considered in a SPF program.

Currently, the list of excludable pathogens for shrimp includes eight viruses, certain classes of parasitic protozoa, and helminth parasites. OI has honored the spirit of the ICES guidelines in establishing founder stocks for its breeding program. To date, OI has obtained SPF populations of L. vannamei from six different regions of its natural range, including Mexico, Ecuador, and Panama. New populations will be added to the program in the future to increase the genetic diversity of the breeding stocks.
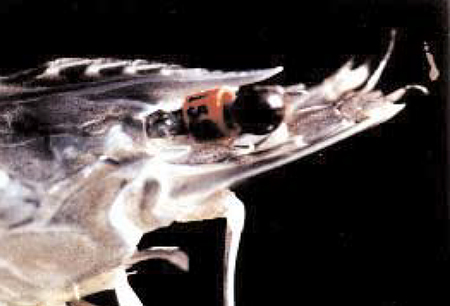
OI maintains a nucleus breeding center at its Makapu’u, Hawaii facility where SPF L. vannamei broodstock are used to produce 80 genetically distinct families twice a year. Broodstock are selected based on a breeding value assigned to the family from which they belong (Fig. 1). A female from a specific family is mated with a specific male by artificial insemination (Fig. 2). In order to minimize the potential deleterious effects of inbreeding, there are no sibling or cousin matings. Following insemination, the female is placed in an individual spawning tank where fertilized eggs are liberated. Viable nauplii are then transferred to individual containers in an indoor hatchery facility where they are reared to postlarvae. Postlarval shrimp are transferred outdoors to individual nursery tanks where they are grown to about one gram, at which time they are injected with an internal, elastomer tag to identify the family from which they came (Fig. 3). Once the shrimp are tagged, they can be cultured in a common environment and inferences can be made about family performance.

From 1995 to 1998, shrimp were selected based on an index where equal emphasis was placed on growth and resistance to Taura Syndrome Virus (TSV). Since 1998, two separate lines have been established. One line is selected 70 percent for TSV resistance and 30 percent for growth in flowthrough shrimp ponds, whereas the other line is selected 100 percent for growth in a recirculating raceway. Shrimp have been evaluated for growth at various research and commercial facilities in Hawaii and on the U.S. mainland, whereas disease-challenge tests have been conducted at research labs in Arizona, Mississippi, and South Carolina.
Prior to the inception of OI’s breeding program, there was a paucity of information about quantitative genetics of penaeid shrimp. One of our continuing goals is to generate information about genetic parameters relevant to a breeding program, including estimates of heritability (h2), phenotypic and genetic variation, phenotypic and genetic correlation, and genotype-environment interaction. To date, over 500 families have been evaluated for growth and TSV resistance, and information about h2, phenotypic variation, and genetic correlation of these traits is summarized below.
Heritability describes the percentage of phenotypic variance that is inherited in a predictable manner and is used to determine the potential response to selection. Theoretically, h2 estimates range from zero to one, although calculated values can exceed this range. Traits with h2 estimates approaching one are highly heritable, whereas traits with h2 estimates approaching zero are not heritable. Data generated from OI since 1995 indicate that the mean h2 estimate for weight gain is 0.40 + 0.06. This estimate is considered moderate to high, and significant improvements in this trait should be made through selection. In contrast, the mean h2 estimate for TSV resistance is only 0.09 + 0.03. Estimates of h2 typically are low for fitness traits, such as disease resistance, and phenotypes with h2 estimates less than 0.15 are often difficult to improve by selection.
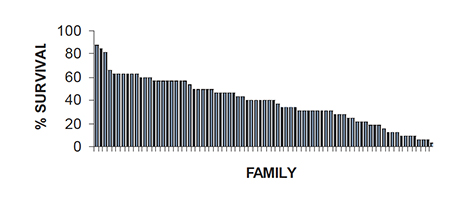
Although h2 estimates for TSV resistance are low, there is high between-family variation in response to a TSV challenge. For example, family survival after 14 days postexposure to TSV per os ranged from 0 percent to 88 percent in a generation of shrimp produced in 1997 (Fig. 4). This degree of phenotypic variation is common in every generation of selectively bred shrimp produced at OI that has been subjected to a TSV-challenge test.
In addition to h2 estimates and information about phenotypic variation, the relationship between TSV survival and harvest weight has been established. Obtaining correlations among commercially important traits is especially important in breeding programs that rely on balanced selection. Results from our research indicate that there is a significant negative correlation between mean family survival in the TSV-challenge test and shrimp harvest weight from a grow-out pond at OI (r = -0.45, p<0.001, n = 513). These results suggest that selecting broodstock based on a balanced selection may result in the loss of valuable alleles both for growth and TSV resistance.
Since the establishment of two separate lines in 1998, the realized response to selection has been high both for growth and TSV resistance. Shrimp selected 100 percent for growth in a recirculating raceway exhibited a 21.2 percent increase in weight gain compared to domesticated, but unselected, control shrimp. Similarly, shrimp selected 70 percent for TSV resistance exhibited an 18.4 percent increase in survival after a TSV challenge compared to control shrimp. These results indicate that significant improvements in shrimp performance can be made by selective breeding. However, it is important to note that the establishment and operation of an SPF shrimp breeding program for multiple traits based on family selection is expensive and requires considerable resources.
Through funding from the USDA and the U.S. Department of Commerce, OI is in the process of building a biosecure nucleus breeding center where we will be able to produce 100 genetically distinct families of shrimp several times a year. Additionally, we will have the capability to operate a SPF breeding program for multiple lines of the same shrimp species or for different species of commercially important penaeid shrimp.
With these resources in place, OI hopes to contribute further to the development of genetically improved shrimp stocks so that U.S. farmers will be able to meet the growing demand for high-quality shrimp products.
(Editor’s Note: This article was originally published in the December 1999 print edition of the Global Aquaculture Advocate.)
Now that you've reached the end of the article ...
… please consider supporting GSA’s mission to advance responsible seafood practices through education, advocacy and third-party assurances. The Advocate aims to document the evolution of responsible seafood practices and share the expansive knowledge of our vast network of contributors.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year.
Not a GSA member? Join us.
Authors
-
Shaun M. Moss
The Oceanic Institute
41-202 Kalanianaole Hwy.
Waimanalo, HI 96795 USA -
Brad J. Argue
The Oceanic Institute
41-202 Kalanianaole Hwy.
Waimanalo, HI 96795 USA -
Steve M. Arce
The Oceanic Institute
41-202 Kalanianaole Hwy.
Waimanalo, HI 96795 USA
Tagged With
Related Posts

Intelligence
Amberjack culture progresses at Oceanic Institute
Amberjacks have excellent aquaculture potential due to their adaptability to conditions of intensive culture, extremely fast growth, and high market value.

Health & Welfare
Biosecurity principles for sustainable production using SPF shrimp
Basic components of biosecurity include knowledge of diseases, adequate detection methods and the use of “clean” shrimp stocks.

Health & Welfare
Born in Hawaii, SPF broodstock shrimp industry faces globalization
The next step for shrimp breeding will be developing animals that aren’t just disease-free, but increasingly resistant to multiple pathogens. The industry is globalizing, with suppliers setting up shop overseas. But its birthplace will always be Hawaii.

Innovation & Investment
Killers at sea: Harmful algal blooms and their impact on aquaculture
The causes and effects of harmful algal blooms have only been studied recently, as damage to the global aquaculture industry mounts.



