Trials conducted with European flat oysters, Japanese oysters and blue mussels

During the last 20 years, the number and size of bivalve hatcheries has dramatically increased to complement declines in wild fisheries and ensure a more consistent, higher-quality supply of material for producers and genetic improvement purposes. The percentage of hatchery-produced animals seeded compared to those caught from wild sources is increasing accordingly. In France, for example, 15 to 20 percent of Crassostrea gigas oyster spat is now produced from hatcheries.
At present, the hatchery rearing of bivalves generally relies on the mass production of microalgae selected based on their mass-culture potential, cell size, digestibility, and overall food value. Bivalves are often fed multispecies algal diets, since they usually support better growth and development than single-species diets.
Algae alternatives
In 1992, Coutteau and Sorgeloos identified the mass production of live algae as a major bottleneck in bivalve hatcheries and nurseries. Algae production comprises up to 30 percent of hatcheries’ operation costs. Furthermore, algae cultures often vary in nutritional value and are subject to seasonal growth patterns and contamination.
Because of the above problems, researchers and culturists are looking for low-cost alternatives. Most of the current alternative diets – such as spray-dried microalgae, microcapsules, yeast- or flour-based diets, lipid microspheres, and emulsions – can not fully replace the live microalgae, but are very useful as partial or backup diets.
New formulations
A range of formulated diets recently developed by INVE Technologies addresses the nutritional needs of bivalves with alternatives to algae. Because the nutritional requirements are very different between oysters, scallops, mussels, and clams, the diets are species-specific.
Priority was given to the development of spat diets, since algae consumption is particularly high at the nursery stage. The spat diets, which are not algae-based and contain various natural ingredients, are dry powders that must be reconstituted with water prior to administration. Almost 80 percent of the particles are smaller than 30 µ.
The diet development included trials with juvenile European flat oysters, Ostrea edulis; Japanese oyster, Crassostrea gigas, spat; and spat of the blue mussel, (Mytilus edulis), and Mediterranean mussel, (Mytilus galloprovincialis), at two commercial bivalve hatcheries: Taylor Resources in Washington, USA, and Roem van Yerseke in the Netherlands. The success with mussel spat led to the release of the first commercial spat diet for mussels, and a spat diet for oysters will be available shortly.
Mediterranean mussel spat

Three weeks post-set diploid mussel spat with an average length of 718 ± 154 µ were collected from a nursery and placed in a flow-through downweller system at the hatchery. Each 7.6-cm-diameter down-weller silo was initially stocked with 3.5 g of seed. The temperature was kept at 18.3 ± 0.7 degrees-C.
A very low level of algae supply (C2, 24 cells per µl) was supplemented with the mussel spat diet at 2.8 percent and 4.3 percent live weight and compared with a C1 optimal diet of 75 cells per µl. For the nonsupplemented algae diets, the total amounts of feed remained constant throughout the course of the experiment at 4.2 and 1.4 g dry-weight algae per day for C1 and C2, respectively. The amount of mussel spat diet, on the other hand, was adapted weekly to the new mussel weights, so the total amount of feed for the supplemented diets increased weekly.
The growth of C2 mussels lagged behind from the start. The C2 + 2.8 percent mussel spat diet and C 2 + 4.3 percent mussel spat diets were not significantly different throughout the experiment, indicating that a supplementation level higher than 2.8 percent did not lead to better results. Both treatments resulted in weight gains almost twice the weight of the C2 diet alone (Fig. 1).
The mussels fed the supplemented C2 diets grew as fast as the animals that received a constant inflow of 75 algae cells per millimeter, so the same result was obtained with only one-third of the algae. This fact becomes even more interesting when one considers that the mussel spat received 95 percent formulated feed and only 5 percent algae on a dry-weight basis by week three.
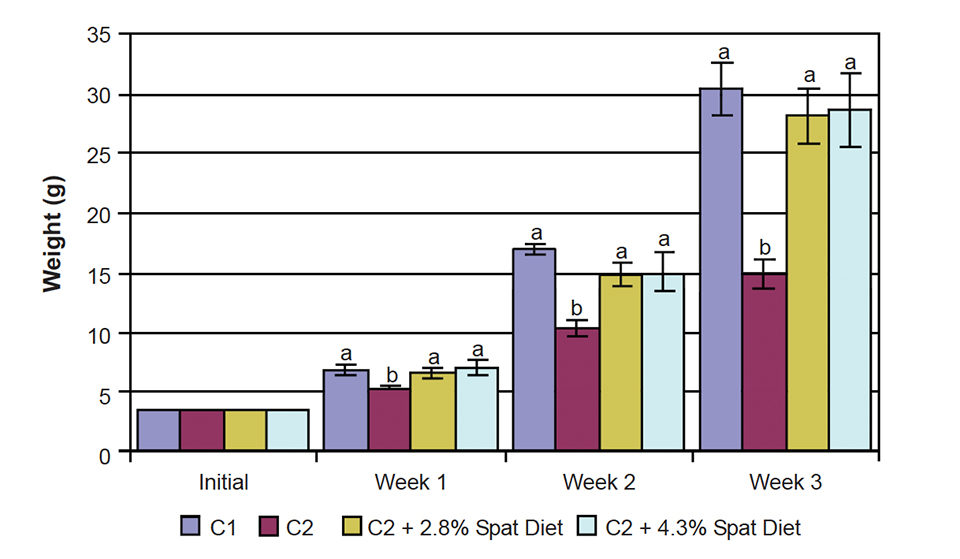
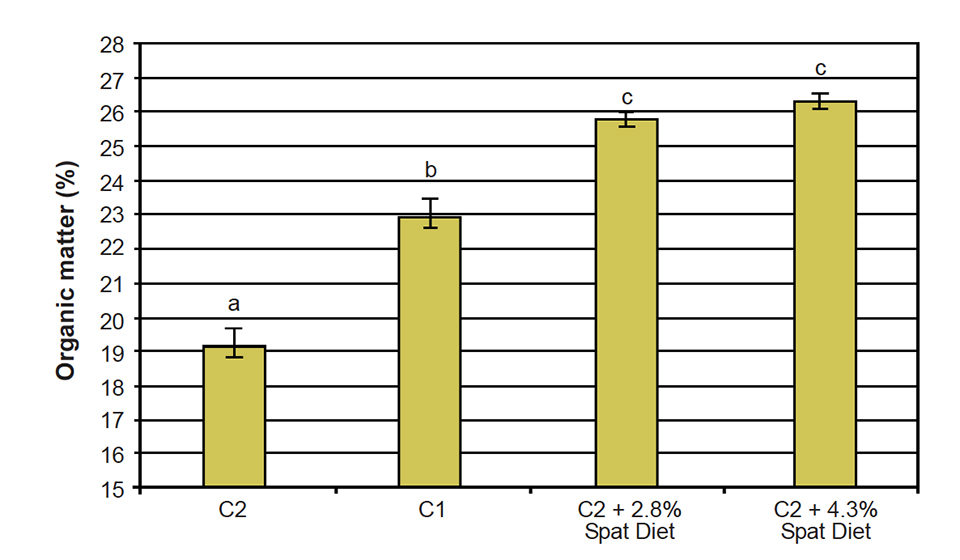
The wet-weight increase obtained with the C2 + 2.8 percent mussel spat diet was 702 percent, while mussels fed C1 had an increase in weight of 768 percent. The increase in wet weight was reflected in an increase of organic matter – not only the shell, but also the soft tissues grew substantially (Fig. 2).
Blue mussel spat
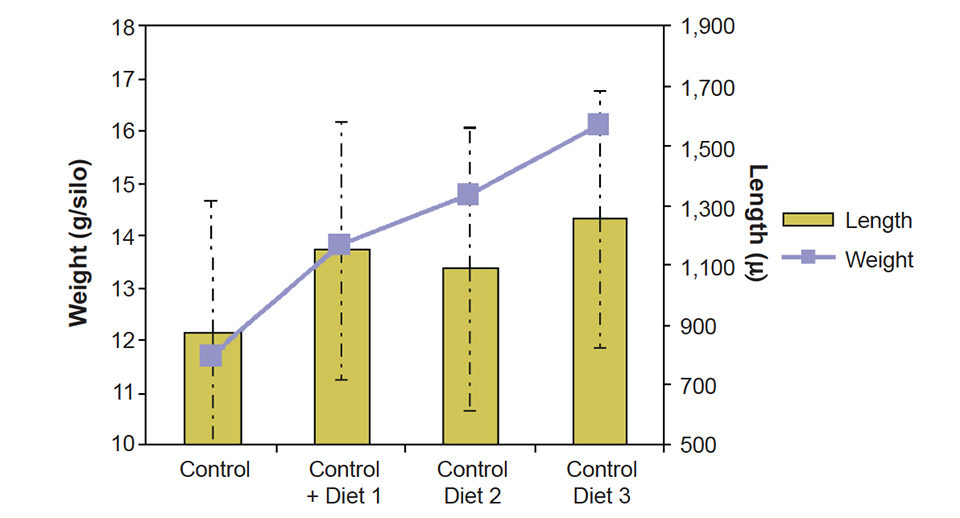
Seven g of blue mussel spat with an average length of 555 ± 208 µ were placed in a closed downwelling system with 40 l of filtered ultraviolet-treated sea water and fed 50,000 Chaetoceros gracilis per milliliter (control) or the algae plus 0.2 g of different INVE formulated diets per week.
After two weeks, the spat was collected and measured. The 130 percent increase in weight with the added mussel spat diet was nearly twice the 67 percent increase observed with the algae-only control diet (Fig. 3).
European flat oyster juveniles
Approximately 300 g of European flat oyster juveniles with lengths of 3.7 ± 0.6 cm were divided over four replicates in a 450-l tank. A control diet consisted of unfiltered sea water only, while the tank Spat 1 also received a formulated diet at a concentration of 0.4g per gram live weight per week. Water was changed every day, and the tanks were cleaned twice a week with fresh water. The average temperature was 20.7 ± 1.5 degrees-C. Salinity was 29.5 ± 05 grams per liter.
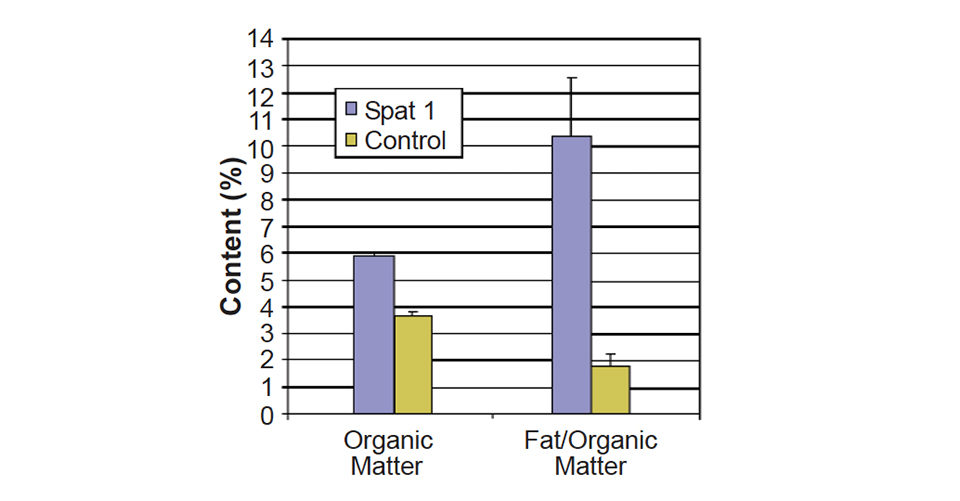
At 0.40 ± 0.05 percent, the daily growth rate over four weeks was twice as fast for animals that received the supplement compared to the control treatment (0.19 ± 0.07 percent). The increase in weight was not due only to shell growth, but an increase in flesh, as the organic content of the oysters increased from 3.7 ± 0.1 percent for the control to 5.9 ± 0.1 percent for the oysters fed the supplement. The absolute lipid and protein contents increased accordingly, but the relative content in lipid also increased significantly when Spat 1 diet was supplemented (Fig. 4).
Editor’s Note: Cited references are available from the author.
(Editor’s Note: This article was originally published in the July/August 2006 print edition of the Global Aquaculture Advocate.)
Now that you've reached the end of the article ...
… please consider supporting GSA’s mission to advance responsible seafood practices through education, advocacy and third-party assurances. The Advocate aims to document the evolution of responsible seafood practices and share the expansive knowledge of our vast network of contributors.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year.
Not a GSA member? Join us.
Author
-
Nancy Nevejan, Ph.D.
R&D Engineer Aqua
INVE Technologies, N.V.
Hoogveld 93
9200 Dendermonde, Belgium
Tagged With
Related Posts

Intelligence
‘Spatiotemporal patterns’ indicate improving perceptions of aquaculture
A study led by University of California Santa Barbara researchers has found that public sentiment toward aquaculture improves over time, a potentially important development with growing interest in offshore aquaculture.

Innovation & Investment
Acoustic control improves feeding productivity at shrimp farms
In systems recently developed for shrimp farms, passive acoustic-based technology enables sensor-based control of multiple automatic feeders. Improved growth and feed conversion have been recorded at commercial farms using the technology.

Responsibility
Ailing waterways hail the oyster’s return
The Lower Hudson Estuary and Chesapeake Bay, two waterways once home to thriving oyster beds, would welcome the shellfish’s return. Aquaculture initiatives in both areas aim to reinvigorate the water and the communities they support.

Intelligence
An emerging shellfish farming industry in Namibia
For shellfish farming in Namibia to continue expanding, industry must better comply with approved sanitation standards. The Namibian Shellfish Monitoring and Sanitation Program, currently in development, will help.



