Hypersalinity leads to increased feed intake, results in slower growth

Farming of penaeid shrimp in earthen ponds takes place in a wide range of water salinities. In grow-out ponds, water salinity can vary from less than 1 to more than 50 ppt. In commercial operations, salinity fluctuates mainly as a response to the dry and rainy seasons, but other factors, such as pond depths, water exchange rates and influences of water pumping also play a role in salinity levels. Shrimp farming under salinities above 40 ppt is quite common, especially in ponds that have been converted from salt pans.
The Pacific white shrimp (Litopenaeus vannamei) is a euryhaline species able to tolerate a wide range of salinity levels. However, this capacity is decreased under hypersaline conditions above 40 ppt. The ideal salinity for the rearing of L. vannamei is around 20 ppt, a value nearer the 24 ppt isosmotic point for this species. In practical culture conditions, hypersalinity leads to increased feed intake and results in slower shrimp growth, poor feed conversion and, at times, lower survival.
Omega-3 (n-3) fatty acids are structural components of cell membranes. They are involved in osmoregulation processes and affect the permeability of cell membranes. Especially in marine species, the highly unsaturated fatty acids (HUFAs) are known to mitigate salinity effects by improving osmoregulation capacity.
The authors conducted a study to evaluate the effects of three lipid sources on the growth performance, resistance and tail fatty acid profiles of L. vannamei reared under hypersalinity conditions.
Trials
Three diets were formulated to contain fish oil, soybean oil and krill oil as their main lipid sources. Except in regards to their essential fatty acid profiles, the diets presented similar nutritional levels, with 35.3 ± 0.13 percent crude protein, 8.8 ± 0.67 percent crude fat, 1.14 ± 0.24 percent crude fiber and 4,222 ± 46.1 kcal/kg gross energy (Table 1).
Nunes, Main lipid sources, inclusion levels and fatty acid composition, Table 1
| Chemical Composition | Fish Oil Diet | Soy Oil Diet | Krill Oil Diet |
|---|
Chemical Composition | Fish Oil Diet | Soy Oil Diet | Krill Oil Diet |
|---|---|---|---|
| Inclusion of Lipid Sources | |||
| Fish oil (g/kg diet, wet basis) | 26.7 | 0 | 0 |
| Krill oil (g/kg diet, wet basis) | 0 | 0 | 48.3 |
| Soybean oil (g/kg diet, wet basis) | 10 | 34.5 | 4.5 |
| Cholesterol (g/kg diet, wet basis) | 0 | 1.3 | 0 |
| Soybean lecithin (g/kg diet, wet basis) | 15 | 15 | 0 |
| Marine:plant oil ratio | 2.7 | 0 | 11 |
| Nutrient Levels | |||
| Total lipid content (g/kg diet, wet basis) | 88.8 | 94 | 80.8 |
| DHA (% total lipids) | 2.54 | 0.3 | 1.6 |
| EPA (% total lipids) | 5.1 | 0.7 | 5.4 |
| HUFA (DHA + EPA) | 7.6 | 0.9 | 6.9 |
| LOA (% total lipids) | 28.3 | 44.7 | 16.2 |
| LNA (% total lipids) | 3.4 | 4.9 | 1.5 |
| EFA (DHA + EPA + LOA + LNA) | 39.4 | 50.5 | 24.7 |
| Peroxide Index | |||
| Total (meq/kg) | 5.18 | 1.8 | 0 |
Researcher Brett Glencross, who studied black tiger shrimp (Penaeus monodon), defined the only data available on penaeid fatty acid requirements. The authors believe that L. vannamei have lower fatty acid requirements than P. monodon, and so designed the fish oil and krill oil diets to meet HUFA levels of at least 80 percent of those required by black tiger shrimp. HUFA levels in the diet with soybean oil were intentionally underformulated as a negative control to compare with the other diets rich in n-3 HUFAs.
For the growth trial, juvenile shrimp of 0.65 ± 0.28 grams body weight were initially stocked in 40 clearwater tanks with 500-L volume at 140 shrimp/m² for a three-week acclimation period. Over this period, shrimp were fed a commercial diet while the tank water salinity was slowly adjusted from 31 ± 1.8 to an ideal 22 ± 0.4 ppt and to 41 ± 0.4 ppt, a high-salinity condition. After acclimation, shrimp stocking density was reduced to 70/m², and the 2.79 ± 0.6 g shrimp were fed the experimental diets twice daily for nine weeks.
For the study on osmoregulatory stress, a model was adopted using salinity increases of 2, 3 and 4 ppt/day for five days with four levels of n-3 HUFAs: 1.1, 2, 2.6 and 3.3 percent of total lipid content.
First, shrimp of 1.71 ± 0.4 g were stocked at a salinity of 29.73 ± 0.9 ppt for a two-week acclimation period in 50 tanks of 500 L. For eight days, shrimp were fed the diet with the lowest n-3 HUFA content. Five days prior to and during salinity increase, shrimp were fed the experimental diets.
Results
Factorial analysis was used to determine the effects of salinity, dietary lipid sources and their interaction. At harvest, no significant (P > 0.05) difference was observed in shrimp survival (93.1 ± 5.2 percent) among shrimp fed the three test diets, regardless of salinity level. Feed intake was significantly (P < 0.05) affected by water salinity and diet type, although a lack of interaction between these factors remained until harvest. Shrimp mean weekly growth was influenced only by diet type (P < 0.05).
Shrimp grew less when fed diets with fish oil or soybean oil – 0.91 ± 0.1 and 0.92 ± 0.1 g/week, respectively – compared to those fed the krill oil diet (1.01 ± 0.1 g/week). Higher water salinity significantly (P < 0.05) depreciated shrimp final body weight when compared to a lower salinity for all diet types. Shrimp fed the krill oil-enriched diet reached final body weights statistically higher than the weights of shrimp fed the fish oil and soybean oil diets (Fig. 1) at both salinity levels.
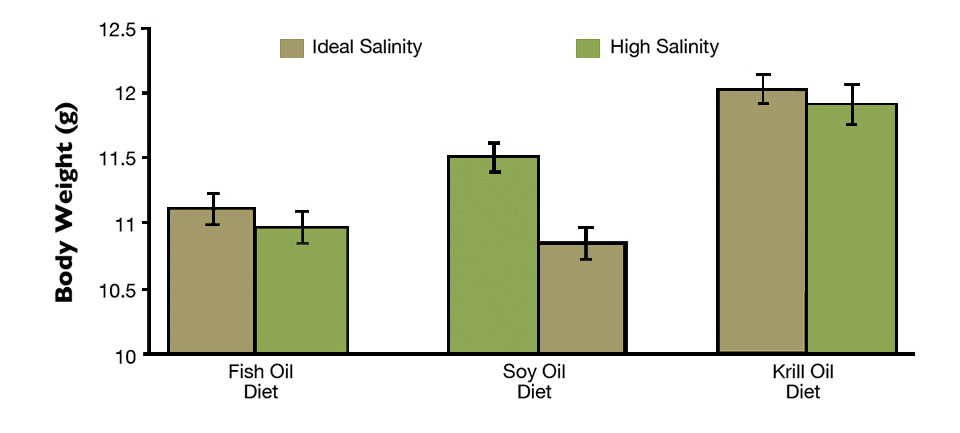
Shrimp yields (553.1 ± 74.4 g/m²) and biomass gains (315.2 ± 42.4 g/tank) were not statistically different among diet type or salinity level. A gas chromatography analysis revealed that the HUFA content of shrimp tails for animals subjected to high-salinity rearing conditions was reduced (Table 2).
Nunes, Fatty acid profiles (% of total lipid content), Table 2
| Fatty Acid | Fish Oil Diet Ideal Salinity | Fish Oil Diet High Salinity | Fish Oil Diet Deviation | Soybean Oil Diet Ideal Salinity | Soybean Oil Diet High Salinity | Soybean Oil Diet Deviation | Krill OIl Diet Ideal Salinity | Krill OIl Diet High Salinity | Krill OIl Diet Deviation |
|---|
Fatty Acid | Fish Oil Diet Ideal Salinity | Fish Oil Diet High Salinity | Fish Oil Diet Deviation | Soybean Oil Diet Ideal Salinity | Soybean Oil Diet High Salinity | Soybean Oil Diet Deviation | Krill OIl Diet Ideal Salinity | Krill OIl Diet High Salinity | Krill OIl Diet Deviation |
|---|---|---|---|---|---|---|---|---|---|
| DHA | 7.13% | 5.34% | -25.08% | 3.69% | 3.53% | -4.36% | 6.55% | 4.22% | -35.59% |
| EPA | 12.42% | 10.09% | -18.78% | 6.61% | 6.6% | -0.08% | 14.96% | 11.5% | -23.15% |
| LOA | 14.64% | 14.93% | 1.99% | 27.31% | 26.94% | -1.36% | 11.63% | 11.1% | -4.58% |
| LNA | 0.69% | 0.63% | -8.48% | 1.21% | 1.3% | 7.01% | 0.67% | 0.54% | -19.37% |
Shrimp fed the fish oil and krill oil diets showed at least 40 percent higher HUFA concentrations in their tails than shrimp fed the soybean oil diet under ideal salinity. Contents of linoleic acid (LOA) and linolenic acid (LNA) in shrimp tails were higher in animals fed the soy oil-enriched feed at both salinity levels. In the osmoreglatory stress study, after a five-day increment in water salinity, final shrimp survival differed in accordance to salinity concentration (P < 0.05).
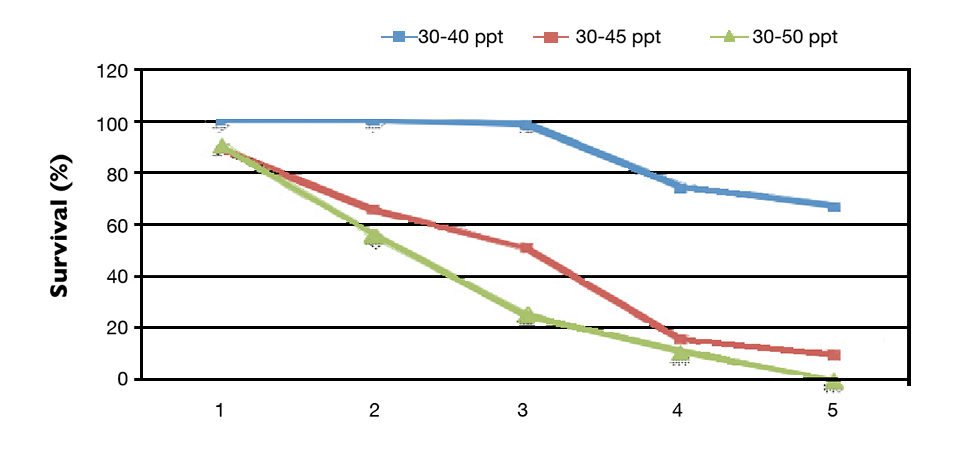
Survival was lower in the treatments subjected to lower increases in water salinity (Fig. 2). In the group exposed to daily salinity increases of 4 ppt, 100 percent shrimp mortality was observed. HUFA supplementation levels provided no benefit to shrimp survival. There was no significant interaction between water salinity and HUFA levels for shrimp survival.
Perspectives
This study showed that lipid source and docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) levels in the diet can influence the growth performance and tail fatty acid profile of L. vannamei farmed under hypersalinity conditions. The diet with krill oil supplementation was found to promote better shrimp growth under both ideal and high salinities over diets containing fish and soybean oils.
It is noteworthy to point out that the high quality standards (as indicated by peroxide analysis) and the presence of high amounts of astaxanthin in the krill oil may have provided further advantages over the other lipid sources. However, omega-3 HUFA supplementation did not appear to counteract the effects of acute osmoregulatory stress.
Results from the study suggested that supplementation of krill oil can be effective against a persistent hypersalinity condition if farmers adopt a precautionary strategy rather than a reactive one. As salinity generally changes in shrimp farms relative to seasons, hypersaline conditions can be easily anticipated.

(Editor’s Note: This article was originally published in the November/December 2009 print edition of the Global Aquaculture Advocate.)
Now that you've reached the end of the article ...
… please consider supporting GSA’s mission to advance responsible seafood practices through education, advocacy and third-party assurances. The Advocate aims to document the evolution of responsible seafood practices and share the expansive knowledge of our vast network of contributors.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year.
Not a GSA member? Join us.
Authors
-
Alberto J.P. Nunes, Ph.D.
LABOMAR – Instituto de Ciências do Mar
Avenida da Abolição 3207 – Meireles
60.180-165, Fortaleza, Ceará, Brazil -
Otavio S. Castro, M.S.
LABOMAR – Instituto de Ciências do Mar
Avenida da Abolição 3207 – Meireles
60.180-165, Fortaleza, Ceará, Brazil -
Marcelo V.C. Sá, Ph.D.
LABOMAR – Instituto de Ciências do Mar
Avenida da Abolição 3207 – Meireles
60.180-165, Fortaleza, Ceará, Brazil -
Hassan Saby-Neto, M.S.
LABOMAR – Instituto de Ciências do Mar
Avenida da Abolição 3207 – Meireles
60.180-165, Fortaleza, Ceará, Brazil
Tagged With
Related Posts

Intelligence
A brief look at genetically modified salmon
If approved by FDA, fast-growing genetically modified salmon will provide a safe and nutritious product similar to other farmed Atlantic salmon.

Aquafeeds
A look at phospholipids in aquafeeds
Phospholipids are the major constituents of cell membranes and are vital to the normal function of every cell and organ. The inclusion of phospholipids in aquafeeds ensures increased growth, better survival and stress resistance, and prevention of skeletal deformities of larval and juvenile stages of fish and shellfish species.

Aquafeeds
A new nutrient for aquaculture, from microbes that consume carbon waste
Biotechnology firm NovoNutrients aims to produce a line of nutraceutical aquafeed additives as well as a bulk feed ingredient that can supplement fishmeal. Its process includes feeding carbon dioxide from industrial gas to a “microbial consortium” starring hydrogen-oxidizing bacteria.
Intelligence
Adding flavor complexity to farmed barramundi
Organoleptic attributes such as flavor and aroma are among the most important factors that influence consumer acceptability and demand for fish products. Consumers have identified farmed fish as less complex and lacking “sealike” or “sea-fresh” flavors and aromas.



