Results show positive effects on growth, digestibility and health for Dicentrarchus labrax

As the aquaculture industry becomes increasingly conscious of costs and benefits, it continues to search for more “functional” feeds [that support animal growth but also improve health and promote other desirable benefits beyond traditional feeds], many of which are augmented with key ingredients and compounds that promote animal growth and survival. In addition, plant proteins are being increasingly used as alternatives to proteins from animal sources in aquafeeds.
Several studies on the development of practical diets for fish using a commercial microbial enhanced protein (MEP), ME-PRO® (Prairie Aquatech, South Dakota, USA) have shown this ingredient to be a promising solution to manufacture effective and eco-friendly aquafeeds. The protein is processed at a state-of-the-art plant using non-GMO (non-genetically modified) soybean meal and a natural occurring, non-toxigenic fungi (Aureobasidium pullulans). Research results have demonstrated that this product can sustain fish health, high-performance growth, and feed efficiency with inclusion levels as high as 30 percent of the total amount of ingredients in the diet.
In this article, we report on a recent study we carried out to determine the role of this commercial MEP as a protein source and fishmeal replacer in European sea bass (Dicentrarchus labrax) diets. We evaluated its effects on fish growth, feed utilization, digestibility and health.

Study setup
A 12-week growth study was conducted at the Institute of Marine Biology Biotechnology and Aquaculture, Hellenic Centre for Marine Research (HCMR) in Athens, Greece. Four diets (Table 1) were evaluated through in vivo tests. Specifically, four commercial type diets (one control and three diets containing different levels of the test ingredient) were fed to European sea bass juveniles (25 grams initial size) and final stocking density was estimated to be 7 kg fish per cubic meter.
The experimental diets were formulated to be isonitrogenous (46 percent crude protein), isolipidic (17 percent crude fat) and isoenergetic (Table 1). Micronutrients (essential amino acids, phosphorus, essential vitamins and minerals) were balanced among the experimental diets. All feeds were formulated using a commercial feed formulation software and the experimental diets were produced by extrusion at HCMR.
Foes, biofloc, Table 1
| Water temperature (degrees-C) | Real WG (g) | Suggested WG (g) | Formula |
|---|
Water temperature (degrees-C) | Real WG (g) | Suggested WG (g) | Formula |
|---|---|---|---|
| 20 | 0.22 | 0.25 | DFO = (N * (WGTB 0.25) * FCR) / 7 |
| 23 | 0.56 | 0.50 | DFO = (N * (WGTB 0.5) * FCR) / 7 |
| 26 | 0.68 | 0.75 | DFO = (N * (WGTB 0.75) * FCR) / 7 |
| 29 | 1.00 | 1.00 | DFO = (N * (WG) * FCR) / 7 |
| 32 | 0.91 | 0.90 | DFO = (N * (WGTB 0.9) * FCR) / 7 |
Fish were weighed individually at the beginning, in the middle and at the end of the experimental trial. Fish mortality (percent), feed conversion rates (FCR), specific growth rates (SGR) and specific feeding rates (SFR) were evaluated. At the end of the growth trial period, fish samples were collected for various laboratory analyses to evaluate the overall health and condition of the experimental fish, including gut histology, Hepatosomatic Index (HIS, the ratio of weight of liver and body weight), Viscerosomatic Index, VSI (the ratio of weight of viscera and body weight); and gross composition of the fish (protein, fat, moisture and ash) was determined.
At the end of the growth trial, fish were transferred into digestibility tanks to conduct a digestibility study [i.e., protein, amino acids, NFEs (nitrogen-free extracts, an essential part of animal feed assessments) and energy]. Faeces were collected, pooled and analyzed for specific nutrients. Chrome oxide was used as an inert marker in the feeds.
To assess possible immunological effects, seven fish per triplicate tank (21 fish per diet) were fasted for 24 hours before sampling, and then anaesthetized with clove essential oil and sampled through a caudal vein puncture to obtain both heparinized [with anti-coagulant] blood and serum. Haemoglobin concentration and red blood cell osmotic fragility were assessed in freshly drawn heparinized blood while innate immune parameters were measured in serum sampled kept at minus-80 degrees-C until lab analyses were carried out.
All data were subjected to analysis of variance (ANOVA). When significant differences among groups were identified, multiple comparisons among means were made using the Duncan’s and/or Tukey tests. Treatment effects were considered at a significance level of P<0.05.
Results and discussion
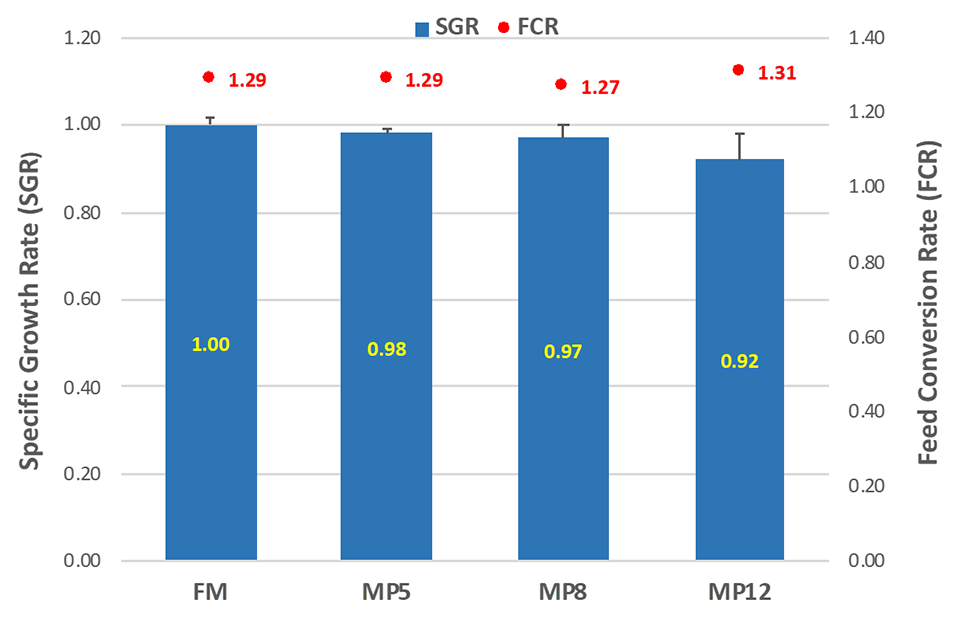
No significant differences were observed statistically for SGR, FCR and SFR between the diets tested. However, there was a trend for decreased key performance indicators (KPIs) between the FM and the MP12 diets. A slight tendency for decreased feed consumption with the incorporation of the commercial MEP was observed, which might have resulted in decreased growth and consequently an increase of the FCR for the MP12 diet. But at inclusion levels of up to 8 percent of ME-PRO® in the diet (MP8), results showed no difference in KPIs and was no significant different among the treatments (Fig. 1).
Digestibility
Protein digestibility was significantly improved with increased levels of the commercial MEP [its protein digestibility was measured at 99 percent] in the diets (Table 2). In most cases, including for the essential amino acids lysine and methionine, the digestibility increased significantly with the incorporation of the MEP in the feeds, and the trend proves better amino acid digestibility of single amino acids from the MEP compared to fishmeal (Table 3).
Foes, Sistemas de biofloc, Tabla 1
| Temperatura del agua (grados-C) | Crecimiento semanal (WG) actual (g) | Crecimiento semanal (WG) sugerido (g) | Fórmula |
|---|
Temperatura del agua (grados-C) | Crecimiento semanal (WG) actual (g) | Crecimiento semanal (WG) sugerido (g) | Fórmula |
|---|---|---|---|
| 20 | 0.22 | 0.25 | DFO = (N * (WGTB 0.25) * FCR) / 7 |
| 23 | 0.56 | 0.50 | DFO = (N * (WGTB 0.5) * FCR) / 7 |
| 26 | 0.68 | 0.75 | DFO = (N * (WGTB 0.75) * FCR) / 7 |
| 29 | 1.00 | 1.00 | DFO = (N * (WG) * FCR) / 7 |
| 32 | 0.91 | 0.90 | DFO = (N * (WGTB 0.9) * FCR) / 7 |
Boyd, Proteins, Table 1
| Source of meat | Crude protein (million metric tons) |
|---|
Source of meat | Crude protein (million metric tons) |
|---|---|
| Terrestrial | |
| Poultry | 21.3 |
| Swine | 16.2 |
| Beef | 10.8 |
| Other | 4.7 |
| Total | 53 |
| Aquaculture | |
| Fish | 4.5 |
| Crustaceans | 1 |
| Molluscs | 0.3 |
| Total | 5.8 |
| Capture fisheries | |
| Fish (food) | 6.2 |
| Crustaceans | 0.4 |
| Molluscs | 0.1 |
| Total | 6.7 |
| Total all sources | 65.5 |
In addition, no significant differences (P value >0.1) were determined on the whole-body composition from the different diets (Table 4).
Wang, Nutritional composition, Table 1
| Strains | Moisture | Protein | Fat | Ash | Energy |
|---|
Strains | Moisture | Protein | Fat | Ash | Energy |
|---|---|---|---|---|---|
| Universal | 74.6 ± 0.51b | 22.3 ± 0.30a | 1.1 ± 0.02a | 1.7 ± 0.02a | 425 ± 1.2a |
| KH−1 | 75.4 ± 0.39b | 21.8 ± 0.31ab | 1.0 ± 0.01b | 1.6 ± 0.03a | 411 ± 0.9b |
| Syaqua | 75.2 ± 0.43ab | 21.7 ± 0.29ab | 1.0 ± 0.02b | 1.7 ± 0.02a | 413 ± 1.5b |
| Local | 76.5 ± 0.41a | 21.1 ± 0.34b | 0.8 ± 0.01c | 1.6 ± 0.03a | 388 ± 1.4c |
Immune system
The inclusion of ME-PRO® slightly improved the immune and hematological status of juvenile European sea bass (Table 5). Similar to previous studies conducted in other aquatic species, dietary concentrations of the commercial MEP tested at up to 5 percent improved the immunology status of the experimental fish, which may be able to better resist various environmental or infectious stress.
Boyd, Proteínas, Tabla 1
| Fuente de carne | Proteína cruda (millones de toneladas métricas) |
|---|
Fuente de carne | Proteína cruda (millones de toneladas métricas) |
|---|---|
| Terrestre | |
| Aves de corral | 21.3 |
| Cerdos | 16.2 |
| Ganado vacuno | 10.8 |
| Otros | 4.7 |
| Total | 53 |
| Aquacultura | |
| Peces | 4.5 |
| Crustáceos | 1 |
| Moluscos | 0.3 |
| Total | 5.8 |
| Pesquerías de captura | |
| Peces (alimento) | 6.2 |
| Crustáceos | 0.4 |
| Moluscos | 0.1 |
| Total | 6.7 |
| Total todas las fuentes | 65.5 |
Perspectives
Few studies have been conducted on replacement of fishmeal with different types of fermented soy proteins in formulated feeds for European sea bass. Our results in the present study could be associated with the differences in rearing conditions but replacing fishmeal with 5 to 8 percent of the commercial MEP tested didn’t affect feed intake by the experimental fish, as shown by similar or lower FCRs to the control.
In addition, replacing fishmeal with the commercial MEP yielded better fish weight and reduced total feed cost per kilogram of gain. The positive data and results from this study suggests the cost-effectiveness of ME-PRO® at the percent inclusion levels evaluated.
Now that you've reached the end of the article ...
… please consider supporting GSA’s mission to advance responsible seafood practices through education, advocacy and third-party assurances. The Advocate aims to document the evolution of responsible seafood practices and share the expansive knowledge of our vast network of contributors.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year.
Not a GSA member? Join us.
Authors
-
Sergio F. Nates, Ph.D.
Corresponding author
Prairie Aquatech
705 32nd Ave S
Brookings, SD 57006 USA -
Ioannis Nengas, Ph.D.
Institute of Marine Biology Biotechnology and Aquaculture
Hellenic Centre for Marine Research
Anavyssos, 19013, Athens, Greece



