Dietary inclusion of 0.4 percent YM improved P. vannamei growth and YM inclusion at 0.2–0.6 percent improved shrimp survival against V. parahaemolyticus

Several research investigations have reported the successful activation of immune-like response through the administration of functional feed additives such as administrating probiotics; nucleotides; beta-glucan and yeast-based additives; seaweed polysaccharides, enzymes and functional immunostimulants. Among these, beta-glucan and yeast-based additives have emerged as promising candidates due to their stable nutritional content and immunomodulatory properties. Yeast is also rich in high-quality protein, making it a valuable dietary supplement for shrimp supplying a balanced source of essential amino acids.
In aquaculture, dietary beta-glucans have been shown to stimulate the immune system of many species by binding specific receptors on immune cells, activating them to defend against pathogens. Research on fish has shown that dietary supplementation with beta-glucans from yeast enhances immune responses.
Meanwhile, in shrimp, the use of beta-glucan could potentiate the humoral innate immune response (immunity that is mediated by macromolecules – including secreted antibodies, complement proteins, and certain antimicrobial peptides – located in extracellular fluids), activate immune-related genes, increase the production of anti-microbial peptides, and improve the agglutination process. The adoption of mannan oligosaccharides (MOS) and beta-glucan yeast-based supplements in shrimp farming can improve aquaculture practices.
This article – summarized from the original publication (Novriadi, R. et al. 2024. Evaluation of dietary yeast derived mannan oligosaccharide for Pacific whiteleg shrimp Penaeus vannamei: Effects on growth performance, immune response, hepatopancreas morphology and resilience to infection against Vibrio parahaemolyticus. Aquaculture Reports, Volume 38, October 2024, 102307) – reports on a study to evaluate the effect of a commercial beta-glucan and a mannan oligosaccharide, MOS, supplementing a low fishmeal-based diet at 0.2; 0.4; 0.6 and 0.8 percent in Pacific whiteleg shrimp (Penaeus vannamei) by assessing animal growth performance and feed utilization. A subsequent pathological challenge trial was also carried out to evaluate the effect of YM on the survival, immune responses and hepatopancreatic histo-morphological condition of shrimp before and after being challenged by Vibrio parahaemolyticus under controlled exposure.
Study setup
The experimental diets, including the control treatment, were designed to be iso-nitrogenous and iso-lipidic (35 percent protein and 7 percent lipid). The control diet contained 10 percent fishmeal (FM), 44.1 percent de-hulled solvent-extracted soybean meal (SBM), and 8 percent corn protein concentrate (CPC) as the dietary protein sources. The series of five diets were formulated to contain 0, 0.2; 0.4; 0.6; and 0.8 percent of a commercial β-glucan and MOS (YM, YeaMOS, Angel Yeast Co. Ltd, Yichang, Hubei Province, China) as an effective broad-spectrum product with >20 percent purity for beta-glucan and >20 percent purity for MOS, designated as 0.2, 0.4, 0.6 and 0.8 percent YM, respectively.
The growth trials were conducted at the Batam Shrimp Research Development (Batam, Kepulauan Riau, Indonesia). P. vannamei postlarvae were obtained from PT. Maju Tambak Sumur (Kalianda, Lampung, Indonesia) and nursed in a semi-indoor recirculating system for three weeks. Then, shrimp (2.02 ± 0.02 grams initial mean weight) were stocked into 98-liter aquaria with 15 shrimp per tank, equivalent to 150 shrimp per meter square. Six replicate groups of shrimp received different experimental diets for 60 days and fed by hand four times daily.
The challenge test followed the growth trials and was carried out at the Department of Aquaculture, Faculty of Agriculture, Gadjah Mada University, Indonesia after 14 days of a feeding period with yeast-containing feed. Just before and after the challenge test, blood and tissue samples were collected to assess the immune status of the shrimp.
For detailed information on the experimental design, animal husbandry and diet formulation and preparation; and data collection and analyses, refer to the original publication.
Results and discussion
Our investigation targeted the application of a commercial yeast cell wall extract rich in mannan oligosaccharides, YM, and beta-glucan with powerful biotic effects confirmed in wider animal studies. There has been a paucity of information for shrimp species and certainly few studies relating to disease and prophylactic intervention through dietary inclusion of these additives. The attributes of MOS and glucan supplementation have been noted in numerous studies with several species of cultured fish, and most investigations have shown positive results leading to improved growth rates and feed utilization efficiency with superior health indicators and often increased survival either in feed trials or post-trial infection studies.
Mortality of shrimp occurred starting from 24 hours post-infection with signs of weakness, displaying passive swimming on the surface, milky white abdominal muscles, anorexia, reddish-yellow coloration of the hepatopancreas, and reduced growth rate. Shrimp fed with 0.4 percent YM had the greatest (P < 0.05) survival rate with 0.8 and 0.6 percent YM being intermediate and control together with 0.2 percent YM having the lowest out of the five experimental treatments.
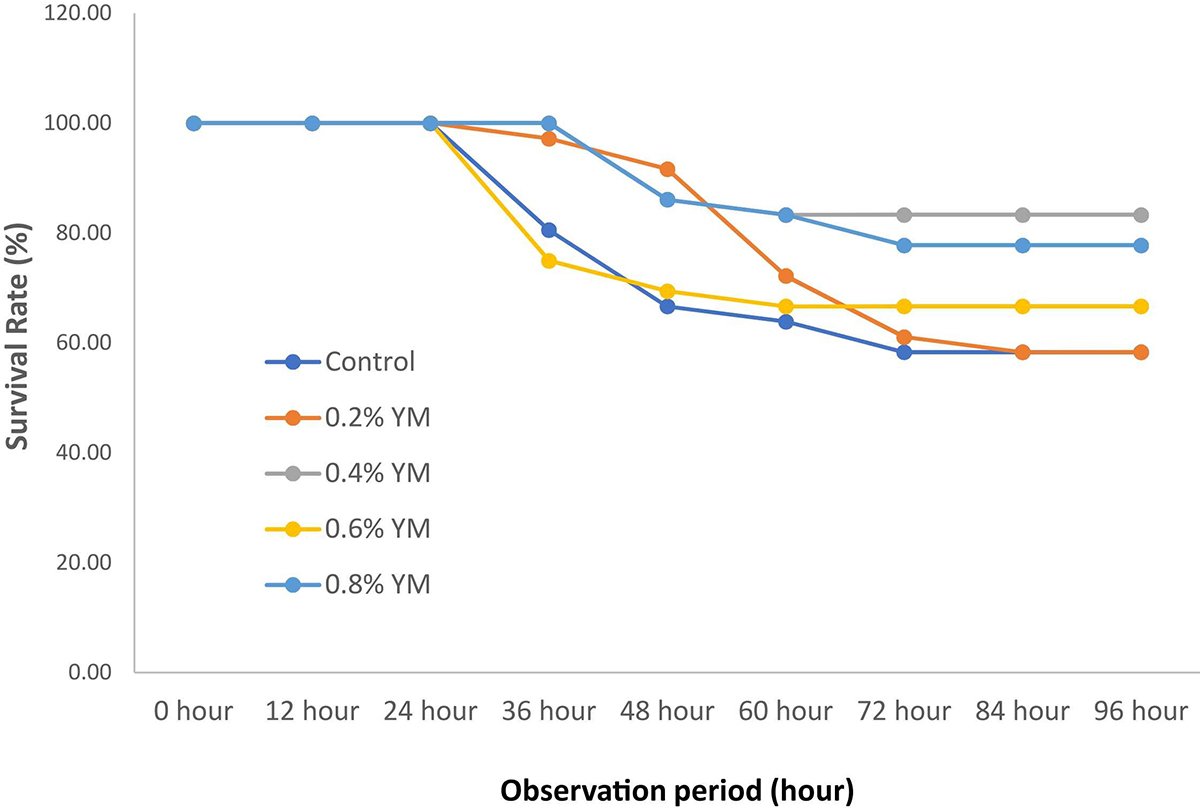
Our results agree with these and other scientific findings measuring similar parameters and indices of health status in farmed shrimp. Numerically, the final body weight (FBW), thermal growth efficiency (TGC) and percentage weight gain (PWG were better in the group of P. vannamei shrimp fed with YM compared to the control treatment despite there being no statistical differences detected. Moreover, the survival rate and biomass in the group of shrimp fed with YM were estimated to be significantly higher compared to the shrimp fed with the control diet.
Mannan oligosaccharides (MOS) derived from yeast cell walls have gained significant attention as a dietary supplement in aquaculture due to their potential to improve gut health, modulate the intestinal microbiome, enhance the innate immune response, and promote growth in fish and for shrimp. MOS in the diet of fish and shrimp can effectively act as prebiotics, promoting the growth and activity of beneficial gut bacteria such as Lactobacillus and Bifidobacterium.
Improving the health and Fusarium resistance of Pacific white shrimp through dietary supplementation
These microorganisms help establish and maintain a balanced gut microbiome, which is essential for nutrient absorption and overall gut health. The promotion of beneficial bacteria can lead to the production of short-chain fatty acids (SCFAs) like butyrate, which can strengthen the gut mucosal barrier. MOS may also have a role in modulating the innate immune response in fish and shrimp and binding to pathogenic bacteria and preventing their adhesion to the gut epithelium. This reduces the colonization of harmful microorganisms in the intestinal tract, which can decrease the incidence of infections. Additionally, MOS may stimulate the activity of immune cells such as macrophages and neutrophils. In combination with probiotics, MOS could act synergistically to enhance the commensal bacterial colonization to greater effect in shrimp although not determined in the current investigation.
MOS and beta-glucan have been shown to enhance the survival rate of shrimp after being challenged with a specific pathogen. Some studies suggest that the supplementation of MOS and beta-glucan could enhance the immune responses and improve the resistance of L. vannamei against V. parahaemolyticus infection.
The results of the present study indicate that shrimp fed with 0.4 percent YM had a higher survival rate, while the treatment with 0.6 and 0.8 percent YM being intermediate compared to the control treatment. If we observe the pattern, shrimp treated with an optimum dose of 0.4 percent YM could maintain the survival rate for 100 percent up to 36 hours post-immersion. After that, mortality occurred with increasing signs of weakness, passive swimming on the surface, milky white abdominal muscles, anorexia, and poor feeding responses. This is in line with other studies that showed that using MOS + beta-glucan less than or equal to 0.4 percent activated the immune responses and resistance against vibriosis also in P. vannamei.
In this research, all shrimp receiving YM treatment and before the infection with V. parahaemolyticus at final dose of 104 cells per mL appeared to have a normal condition compared to the control treatment. However, after being infected, all shrimp exhibited severe necrotic cells and massive sloughing of hepatopancreatic tubule epithelial cells into the lumen.
In line with our study, other researchers reported that the use of yeast culture in the range of 5.25–10.52 percent to replace the inclusion of FM from 4–8 percent in the diet formulation had no significant effects on the hepatopancreas of shrimp. However, when shrimp are infected with V. parahaemolyticus, the hepatopancreas will undergo symptoms characterized by shedding of epithelial cells within the hepatopancreas, followed by necrosis of epithelial cells and hemocytic infiltration at later stages.
Our current histopathological results suggest that the dietary treatment of YM maintained the integrity of the hepatopancreas within an ideal condition period. Meanwhile, shrimp showed severe structural damage after massive exposure to V. parahaemolyticus at a later evaluation time where infection had become more established in tissues and organs and beyond dietary intervention.
Perspectives
The results of this study with P. vannamei shrimp – and other scientific evidence – support incorporating yeast-based products into shrimp diets to improve the biomass yield and immune function against Vibrio parahaemolyticus. A dietary inclusion level of 0.4 percent could be considered as an optimum dose, as it provides the optimum responses for biomass gain, survival rate, phagocytic activity and index, and survival rate of shrimp after being challenged with V. parahaemolyticus, before and after infection. These results are relevant to shrimp feeds with reduced fishmeal and higher plant by-product inclusions that can hinder shrimp resilience under stressful intensive production systems.
Now that you've reached the end of the article ...
… please consider supporting GSA’s mission to advance responsible seafood practices through education, advocacy and third-party assurances. The Advocate aims to document the evolution of responsible seafood practices and share the expansive knowledge of our vast network of contributors.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year.
Not a GSA member? Join us.
Author
-
Romi Novriadi, Ph.D.
Corresponding author
Department of Aquaculture, Jakarta Technical University of Fisheries (Politeknik Ahli Usaha Perikanan), Ministry of Marine Affairs and Fisheries, Republic of Indonesia, Jl. Raya Pasar Minggu, Jati Padang, Jakarta 12520, Indonesia



