Prudent managers measure dissolved oxygen concentration at night

Although molecular oxygen comprises about 21 percent of atmospheric gases, its concentration in waters of aquaculture facilities can be unacceptably low for good survival and growth of culture species. Like other atmospheric gases, oxygen has a characteristic solubility, and its dissolution in water is directly proportional to its percentage composition and partial pressure in the atmosphere.
The pressure of oxygen in air above a water surface forces oxygen molecules into the water until the pressure of oxygen in the water equals that in the air. This state is known as oxygen saturation. Molecular oxygen contained in water is called dissolved oxygen (D.O.).
Concentration in water
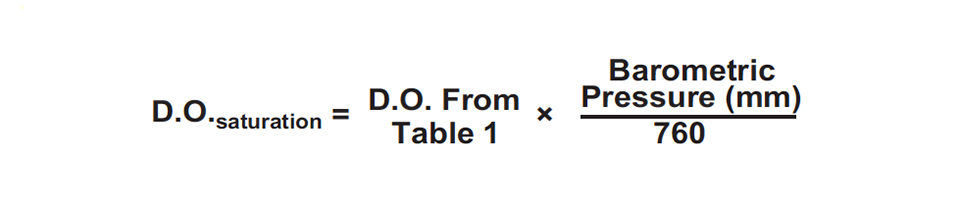
The D.O. concentration, usually expressed as a weight/ volume relationship such as milligrams per liter, at saturation decreases with increasing water temperature and salinity (Table 1), and also as barometric pressure falls. The following equation may be used to convert D.O. concentrations for standard pressure to those at saturation for other barometric pressures.
Boyd, Solubility of oxygen (mg/l) as a function of temperature and salinity, Table 1
| Temperature (° C) | Salinity (ppt) 0 | Salinity (ppt) 5 | Salinity (ppt) 10 | Salinity (ppt) 15 | Salinity (ppt) 20 | Salinity (ppt) 25 | Salinity (ppt) 30 | Salinity (ppt) 35 | Salinity (ppt) 40 |
|---|
Temperature (° C) | Salinity (ppt) 0 | Salinity (ppt) 5 | Salinity (ppt) 10 | Salinity (ppt) 15 | Salinity (ppt) 20 | Salinity (ppt) 25 | Salinity (ppt) 30 | Salinity (ppt) 35 | Salinity (ppt) 40 |
|---|---|---|---|---|---|---|---|---|---|
| 0 | 14.602 | 14.112 | 13.638 | 13.180 | 12.737 | 12.309 | 11.896 | 11.497 | 11.111 |
| 5 | 12.757 | 12.344 | 11.944 | 11.557 | 11.183 | 10.820 | 10.470 | 10.131 | 9.802 |
| 10 | 11.277 | 10.925 | 10.583 | 10.252 | 9.932 | 9.621 | 9.321 | 9.029 | 8.747 |
| 15 | 10.072 | 9.768 | 9.473 | 9.188 | 8.911 | 8.642 | 8.381 | 8.129 | 7.833 |
| 20 | 9.077 | 8.812 | 8.556 | 8.307 | 8.065 | 7.831 | 7.603 | 7.832 | 7.167 |
| 25 | 8.244 | 8.013 | 7.788 | 7.569 | 7.357 | 7.150 | 6.950 | 6.754 | 6.565 |
| 30 | 7.539 | 7.335 | 7.136 | 6.943 | 6.755 | 6.572 | 6.394 | 6.221 | 6.052 |
| 35 | 6.935 | 6.753 | 6.577 | 6.405 | 6.237 | 6.074 | 5.915 | 5.761 | 5.610 |
For example, suppose the water temperature in a freshwater pond is 20 degrees C and the barometric pressure is 710mm. The D.O. concentration at saturation is 8.48mg/l – about 0.6 mg per liter less than at 760 mm pressure.
Daily changes in barometric pressure are relatively small and have effects of only 0.1 or 0.2 mg per liter on dissolved oxygen concentration. Barometric pressure differs greatly with changes in elevation. The solubility of D.O. at 20 degrees-C in freshwater is 9.08 mg per liter at sea level, but only 7.19 mg per liter at 2,000 meters, where the pressure averages 608 mm.
The D.O. concentration at saturation increases with increasing water depth because hydrostatic pressure adds to the barometric pressure acting on the water surface.
Concentration variations
Waters do not attain D.O. saturation and remain in this state. The main reason for variations in D.O. in natural waters is photosynthesis by aquatic plants and respiration by all aquatic organisms. These processes cause D.O. concentration to change faster than the diffusion of oxygen between air and water can maintain a saturated state. Typically, water in aquaculture ponds has D.O. concentrations above saturation during much of the day that then drop below saturation during nighttime.
Molecular oxygen diffuses from water to air when D.O. levels exceed saturation and from air to water when the concentrations are less than saturation. The difference in D.O. concentration and saturation is the D.O. deficit. The greater the deficit, the larger is the tendency for oxygen to exchange across the water surface. Moreover, turbulence of the water surface from wind or splashing over rocks results in more rapid oxygen exchange.
The amount of dissolved oxygen in water sometimes is expressed as percentage saturation according to the following equation.
Effects on aquaculture species
In ponds, surface water often is less than 50 percent saturated with D.O. in the early morning and above 200 percent saturation by mid-afternoon. D.O. supersaturation seldom is harmful to fish or shrimp. Supersaturation of water with air, however, can be a major problem in trout or other hatcheries by causing gas bubble trauma.
The effects of D.O. concentration on aquaculture species usually are reported on a concentration basis for the optimum temperature range of the species under consideration (Table 2). In reality, aquatic animals respond to the pressure of oxygen in the water instead of D.O. concentration.
Boyd, Effects of dissolved-oxygen concentration, Table 2
| Dissolved Oxygen (mg/l) | Approximate Percentage Saturation at 20° C | Effect |
|---|
Dissolved Oxygen (mg/l) | Approximate Percentage Saturation at 20° C | Effect |
|---|---|---|
| 0-0.3 | 0-4 | Small fish survive short exposure |
| 0.3-1.0 | 4-13 | Lethal if exposure prolonged |
| 1.0-5.0 | 13-64 | Fish survive, but growth slow for prolonged exposure |
| 5.0-Saturation | 64-100 | Desirable range |
D.O. management
Management of D.O. in ponds with static water is difficult, for plankton, benthos, and other organisms use more D.O. than is used by the culture species. Managers of nonaerated ponds usually limit stocking and feeding rates to those that have proven safe in the past. In channel catfish farming, for example, feed inputs to non-aerated ponds are limited to 30 to 40 kg per hectare (ha) per day.
Prudent managers measure dissolved oxygen concentration at night and apply emergency aeration with tractor-powered, emergency aerators when necessary. Aeration of channel catfish ponds with electric aerators allows daily feed inputs above 100 kg per ha and up to fourfold more production than that possible in non-aerated ponds.
When aerators are operated during the daytime, they increase the loss of oxygen to the air if pond water is supersaturated with D.O. Aerators usually are most effective in transferring oxygen to water during nighttime. In ponds, the culture species must compete with other organisms for the added D.O. Studies of channel catfish ponds suggested that only 15 to 25 percent of the D.O. provided by aerators is used by the culture species.
Flow-through, cage systems
In flow-through aquaculture systems, rapid water flow prevents the growth of plankton and flushes out wastes. Nearly all the D.O. in inflowing water is available to fish. Stocking and feeding rates in trout raceways are established so that D.O. concentration does not fall below 5 mg per liter and stress the fish.
Suppose that water containing 10 mg per liter D.O. enters a raceway at 10 cubic meters per minute. The water supply has 5 mg per liter of available D.O. or 72 kg D.O per day (5 grams/ m3 × 10 m3/minute × 1,440 minutes/day × 10-3). It usually is possible to apply 5 kg feed for each kilogram of D.O., so 360 kg feed can be applied daily. The fish biomass necessary to fully use the available D.O. at a feeding rate of 3 percent body weight daily would be 12,000 kg (360 kg feed/day ÷ 0.03).
The steps in the calculation above can be incorporated into the following equation for calculating the carrying capacity of flow-through systems.
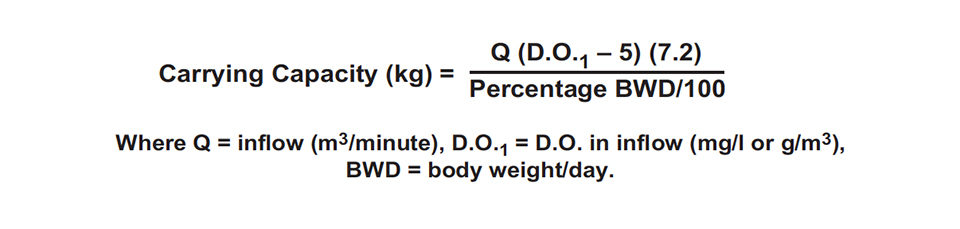
Aeration also can be used to increase carrying capacity in flow-through systems. However, in raceways, almost the entire amount of D.O. supplied by aeration would be available to the fish, for there are few other organisms present to use it.
In cage and net pen culture, or culture of bivalve shellfish, the dissolved oxygen concentrations in the water result from natural conditions and usually cannot be influenced by management. Sites for such aquaculture projects should provide high-quality water and good circulation. Many cage culture operations have experienced serious losses to dissolved oxygen depletion when thermal destratification of eutrophic lakes occurred.
(Editor’s Note: This article was originally published in the January/February 2008 print edition of the Global Aquaculture Advocate.)
Now that you've reached the end of the article ...
… please consider supporting GSA’s mission to advance responsible seafood practices through education, advocacy and third-party assurances. The Advocate aims to document the evolution of responsible seafood practices and share the expansive knowledge of our vast network of contributors.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year.
Not a GSA member? Join us.
Author
-

Claude E. Boyd, Ph.D.
Department of Fisheries and Allied Aquacultures
Auburn University
Auburn, Alabama 36849 USA
Tagged With
Related Posts

Responsibility
A look at unit processes in RAS systems
The ability to maintain adequate oxygen levels can be a limiting factor in carrying capacities for RAS. The amount of oxygen required is largely dictated by the feed rate and length of time waste solids remain within the systems.

Responsibility
Advances in super-intensive, zero-exchange shrimp raceways
Research at the Texas AgriLife Research Mariculture Laboratory is investigating ways to improve the economic viability of super-intensive raceways for shrimp production.

Aquafeeds
Algae alternative: Chlorella studied as protein source in tilapia feeds
Chlorella and other species have potential as protein sources in aquafeeds. In trials with tilapia fry raised in a recirculating system, the fish received a fishmeal-based control diet or feeds with portions of the fishmeal replaced by Chlorella.

Health & Welfare
Ammonia toxicity degrades animal health, growth
Ammonia nitrogen occurs in aquaculture systems as a waste product of protein metabolism by aquatic animals and degradation of organic matter, or in nitrogen fertilizers. Exposure can reduce growth and increase susceptibility to diseases in aquatic species.



