It is not sufficient to certify the exotic animals as “pathogen-free” for a list of known pathogens with available diagnostic tests

Part 1 of this article series contended that the greatest risks for the spread of diseases in aquaculture lies with the careless, cross-boundary movement of culture animals and the limited ability of current regulations to control such movement. Failure to appreciate the dangers of animals translocations has resulted in widespread shrimp epizootics such as Taura Syndrome and White Spot Syndrome.
https://www.aquaculturealliance.org/advocate/dangers-of-viral-pathogens-in-translocated-shrimp-part-1/
Taura syndrome
Taura Syndrome was first described as a shrimp disease in Ecuador in 1992. Both toxic and infectious etiologies were considered. An infectious agent subsequently described in 1995 was named Taura Syndrome Virus or TSV. However, the authors of the original Taura report disputed that TSV was the cause and recommended that the virus be called Infectious Cuticular Epithelial Necrosis Virus. Here the virus will be referred to as TSV.
TSV is a cytoplasmic, nonenveloped icosahedral virus that is now classified in the family Dicistroviridae near to cricket paralysis virus.
TSV was a serious cause of shrimp mortality for reared Pacific white shrimp, (Penaeus vannamei), in the Americas, where it spread principally through regional and international transfer of live postlarvae and broodstock. More recently, it was reported in P. vannamei reared in Taiwan after the importation of live shrimp stocks from the Americas. A similar scenario in Thailand led to the first TSV outbreaks in late 2002, probably originating from shrimp imported for aquaculture from Taiwan and China.
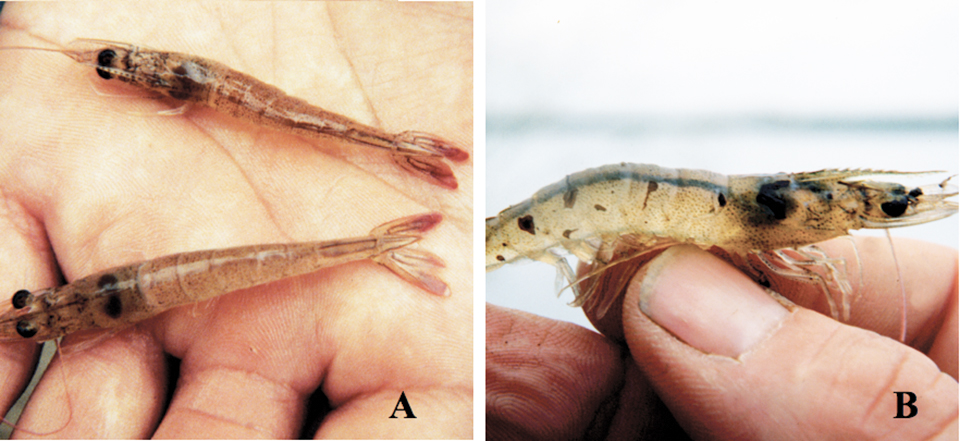
Although TSV infects a number of penaeid species, it has caused serious commercial losses only for the juvenile to adult stages of P. vannamei. Disease caused by TSV has three distinct acute, transition, and chronic phases, although only the acute and transition phases show distinguishable gross signs.
Gross signs for moribund P. vannamei in the acute phase include overall pale reddish coloration and distinctly red tail fans and pleopods, where focal necrosis of the cuticular epithelium can be seen with a 10-x hand lens. These shrimp also show gross signs of soft shells and empty guts, and usually die during molting.
The transition phase in TSV epizootics shows gross signs of random, multifocal, irregularly shaped melanized cuticular areas that mark resolving TSV lesions. Affected shrimp may or may not have soft cuticles and red coloration, and may behave and feed normally. If shrimp with these black lesions survive the next molt, the lesions disappear and the animals appear normal despite the continuing presence of the virus, especially in the lymphoid organ. Thus, TSV survivors can grow to adults as grossly normal animals and produce postlarvae that also appear normal.
White spot syndrome
Although White Spot Syndrome Virus (WSSV) initially caused serious shrimp production losses in Asia only, it must now be considered the single most serious shrimp pathogen worldwide. It was first reported in farmed (Penaeus japonicus) in Japan in 1993, and called Penaeid Rod-Shaped DNA Virus or Rod-Shaped Nuclear Virus of P. japonicus. Similar rod-shaped viruses from elsewhere in Asia were called by various names, but Donald Lightner grouped them in a single WSSV complex. WSSV is now included in the new virus family Nimaviridae and new genus Whispoviridae.
Captured broodstock and fry used to stock rearing ponds are known to carry WSSV, as are numerous other crustaceans and perhaps even aquatic insect larvae, but massive mortality usually occurs with juvenile shrimp in rearing ponds, probably precipitated by environmental factors.
In Thailand, outbreaks of White Spot Syndrome in shrimp culture ponds initially occurred in 1994 and caused a peak estimated lost production of 70,000 metric tons (MT) in 1996. The total cumulative lost production for all Asian countries since 1993 must now amount to more than a million MT, with the world loss much higher.
The WSSV outbreaks in Japan were the first widely reported, but they actually followed Chinese outbreaks and apparently resulted from the import of grossly normal postlarvae from China directly to aquaculture facilities in Japan. No one knows how the virus spread throughout Asia after that, but the common practice of moving broodstock and postlarvae freely among countries likely contributed.
Almost certainly, WSSV was spread from Thailand to Malaysia and India in this manner. In addition, WSSV was not reported in the Philippines until 2000, probably because of an effective Philippine government ban on broodstock and postlarvae importation. Anecdotal evidence suggests that the Philippine outbreaks in the late 1990s originated from the illegal importation of postlarvae from China. As in Asia, a good part of the spread of WSSV in the Americas probably resulted from the international transport of live shrimp for aquaculture.
It is curious that WSSV was not reported in China prior to the catastrophic disease outbreaks in 1993, in spite of the fact that shrimp aquaculture had been practiced there for many years. The nature of the outbreaks was reminiscent of the initial Infectious Hypodermal and Hematopoietic Necrosis Virus (IHHNV) outbreaks for (P. stylirostris) in the Americas, suggesting the WSSV may have originated from the importation of a distant, exotic aquaculture species carrying a previously unknown pathogen. Whatever the original source, it is clear that the majority of the subsequent geographical spread was via grossly normal broodstock and postlarvae.

Lessons unlearned
Experience with three large-scale epizootics in shrimp has shown that the spread of disease usually resulted from translocation of grossly normal broodstock or postlarvae. At the initial phase of all three cases, the causal agent was unknown and appropriate diagnostic tools were developed only after the fact.
With IHHNV, the original source of the virus was identified, but for TSV and WSSV, it remains to be determined. However, good guesses might be a jump from a carelessly imported exotic crustacean to P. monodon for the case of WSSV in China and Taiwan, and the jump of a native crustacean pathogen to exotic P. vannamei in the case of TSV in the Americas.
In any case, the take-home lesson was that grossly normal shrimp were capable of carrying one or more unknown viral pathogens without gross or histological signs of disease. Despite this knowledge and the availability of appropriate diagnostic tools, TSV was transferred to China, Taiwan, and Thailand. Obviously, forewarning and existing mechanisms to prevent the transfers failed.
The consequence of TSV transfer to Thailand has been the development of Thai genetic variants that differ from those in the United States and China, and that have been found in native species in addition to exotic P. vannamei. Since virulence differs among the American genetic variants, we might expect the same of those developing in Thailand. The long-term consequences for native species is unknown.
In addition, a new problem called Monodon Slow Growth Syndrome began to occur widely in Thailand in 2002. The rapid, countrywide occurrence of the problem, its tendency to be related to postlarvae batch, and the lack of association with known pathogens suggested the possible occurrence of a new infectious agent. No conclusions can yet be made regarding this speculation, but concurrence of the problem with large-scale import and cultivation of P. vannamei opens the possibility for yet another new, cryptically introduced virus.
Recommendations
Given the propensity of shrimp, crustaceans and other arthropods to carry viral infections, it is reasonable to suggest that International Council for the Exploration of the Seas (ICES) guidelines be modified and closely followed to guard against the possibility of introducing new viral pathogens. It is not sufficient to certify the exotic animals as “pathogen-free” for a list of known pathogens with available diagnostic tests. There is additional danger from previously unknown viruses or variants of known viruses for which no assay methods exist.
Given the experience with IHHNV in P. monodon, it would seem prudent to add to the ICES guidelines a special requirement that native species of shrimp and other economically important crustaceans be included as cohabitants in the quarantine phase of the importation process. This would guard against the unintentional transfer of any well-tolerated, unknown pathogen from the exotic host to local species that might be more vulnerable and more seriously affected. Doing this would have avoided the release of IHHNV in the Americas, WSSV in Japan, and TSV in Taiwan.
However, to make the guidelines work, standard protocols for inspection, disease diagnosis, and certification of shipments of live marine animals would be required, and the infrastructure for this in Asia is not always in place. Most effective would be increased awareness of the potential threats among aquaculture practitioners themselves and their cooperation in guarding against them.
Outright import bans on all crustaceans might be an effective exclusion measure, but would unreasonably deprive aquaculturists of potentially good alternative species for cultivation. A better approach would be the promotion of joint-venture operations with developers of domesticated or genetically improved stocks to establish local breeding centers via the modified ICES protocol. Once safely established, the costly process of continuous importation and screening of stocks could be avoided.
Note: Cited references are available from the author.
(Editor’s Note: This article was originally published in the February 2006 print edition of the Global Aquaculture Advocate.)
Now that you've reached the end of the article ...
… please consider supporting GSA’s mission to advance responsible seafood practices through education, advocacy and third-party assurances. The Advocate aims to document the evolution of responsible seafood practices and share the expansive knowledge of our vast network of contributors.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year.
Not a GSA member? Join us.
Author
-
Timothy W. Flegel, Ph.D.
Centex Shrimp and BIOTEC
Chalermprakiat Building
Faculty of Science
Mahidol University
Rama 6 Road
Bangkok 10400 Thailand
Tagged With
Related Posts

Health & Welfare
The Shrimp Book: Principal shrimp infectious diseases, diagnosis and management
Disease outbreaks in shrimp occur not only due to the presence of pathogens, but also to suboptimal culture conditions and system management.

Health & Welfare
A case for better shrimp nutrition
Shrimp farm performance can often be below realistic production standards. Use proven nutrition, feeds and feeding techniques to improve profitability.
Health & Welfare
Evolutionary history of Taura Syndrome Virus
Based on a phylogenetic analysis with the BEAST program, the authors determined the evolutionary relationships among 83 Taura syndrome virus isolates.

Health & Welfare
Biosecurity principles for sustainable production using SPF shrimp
Basic components of biosecurity include knowledge of diseases, adequate detection methods and the use of “clean” shrimp stocks.



