Greater ability to bind with organic matter from water

In intensive rearing of marine fish, the addition of microalgae to the culture water has a beneficial influence on survival and growth. The effect has mainly been attributed to improved foraging conditions and a beneficial nutritional impact. Suspended particles in the water may also have a significant impact on the microbial environment and as a consequence affect the establishment of the primary microflora of larvae.
Live microalgae have a positive effect on both the microbial environment of the culture water and the initial bacterial colonization of the guts of the culture fish. Microalgae like Nannochloropsis oculata and Isochrysis galbana are therefore typically used to condition rearing water in intensive marine aquaculture. Algae paste and other commercially available concentrates of microalgae, which save labor compared to the production of live algae, are also used to create “greenwater” in larval rearing.
Both live microalgae and algae paste have been substituted by clay in a recently developed first-feeding protocol for the larvae of halibut (Hippoglossus hippoglossus). Clay is more cost effective than algae or algae paste, and has a stable and predictable quality. On the other hand, clay does not contribute to feeding the fish larvae.
Microbial effects
In marine fish larvae culture water, live algae, algae concentrates, and clay particles represent different degrees of organic contribution and associated bacteria and surface properties, and may have different effects on the microbial community. Live algae cultures contain algae cells, associated bacteria, and algal metabolites, including antibacterial compounds. Algae paste is a mixture of weakened cells, cell remnants, organic material, and perhaps algal metabolites.
In contrast to algae, clay contributes little organic or microbial load to fish tanks, and may on the contrary adsorb organic material and perhaps even viruses and bacteria. Whereas algae can serve as food for larvae and live feed, clay has no nutritional value.
The majority of the colloidal suspension in intensive fish culture water consists of bacteria associated with feces and feed particles. The addition of particles to form aggregates, followed by siphoning of aggregates settled on tank bottoms or removal of flocs by surface skimmers may be a means to transport organic wastes and a fraction of the bacteria out of larval tanks.
Water exchange
The opportunity of utilizing particles for bacteria and organic matter removal may especially make a difference during the early larval period of low water exchange rates. Exchange of water removes surplus feed, feces, bacteria, organic matter and waste compounds from rearing tanks.
At low exchange rates, additions of live feed and algae with associated microflora have a relatively larger influence on water quality than at higher exchange rates. The necessity of keeping appropriate live prey concentrations and sensitivity of larvae to mechanical disturbances limits water exchange during the early larval stage.
Cod research
For larvae of cod (Gadus morhua), daily water exchange is often set to one to two times the tank volume during the first week after hatching. Consequently, the larvae benefit relatively little from external water treatment. However, treatment of the culture water already inside the fish tanks by the addition of particles may be a way to reduce levels of bacteria and organic matter.
In a recent experiment conducted as part of the strategic CODTECH research program financed by the Norwegian Research Council, the authors compared the effects of live microalgae, algae concentrate, and clay on the bacterial community and organic matter in larval fish tanks.
Study setup
Five 70-liter tanks with central diffuser aeration and sand-filtered seawater were kept stagnant at 12 degrees-C for 24 hours. The water exchange rate was thereafter set to 200 percent daily. To three tanks were added Isochrysis galbana microalgae at 2 mg C/l, Nannochloropsis oculata algae paste at 2 mg C/l, or clay particles larger than 200 µ at 100 mg/l. All tanks except one negative control received 25 µg yeast extract per milliliter to simulate increased substrate.
The “greenwater” treatments with added live microalgae or algae paste initially had 8.2-8.9 x 104 particles per milliliter in the 3- to 20-µ size range. Algae paste particles settled out of the water column more quickly than live algae and clay and after two hours were reduced to 1.8 x 104 particles per milliliter.
Results
In this study, clay showed greater ability to bind with organic matter from the water than the other particles. The clay transported more organic matter to the tank bottom than live or concentrated microalgae (Fig. 1). In addition, while the particulate organic matter (POC) measured in the clay tank consisted solely of organic matter from the water column, algae cells themselves contributed to the POC measured, which effectively made the difference in transport even larger between the particle types.
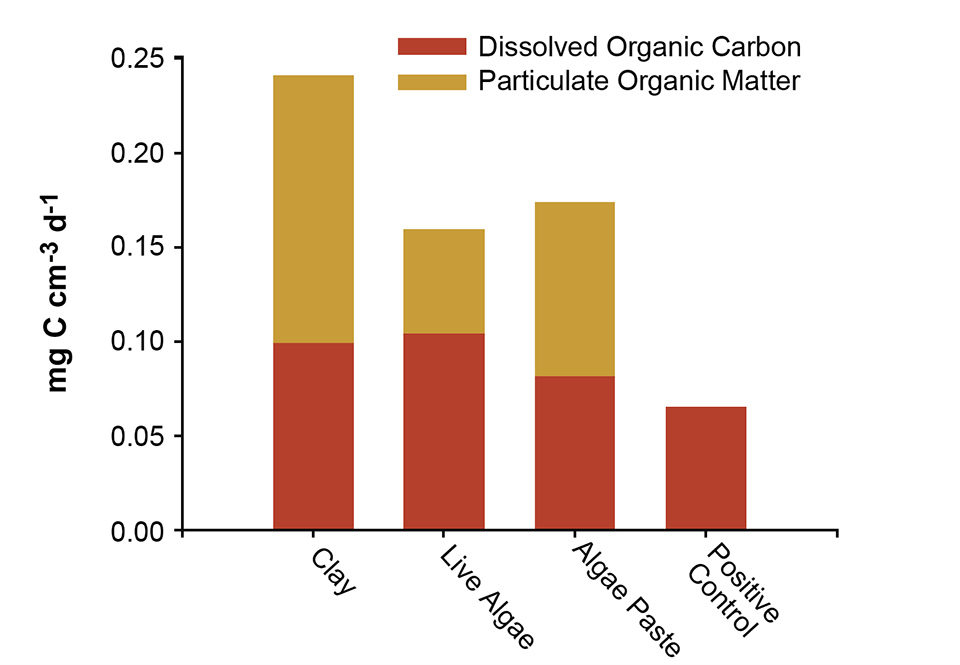
The bacteria numbers in tanks might be expected to increase with increasing dissolved organic carbon (DOC) concentration, as DOC provides substrate for heterotrophic bacteria. If clay can be used to transport DOC out of the water column, it may reduce the potential for bacterial growth in the first feeding tanks.
All techniques reduced initial bacterial proliferation after nutrient shift-ups. The bacterial community of the relatively low-temperature water immediately responded to the induced nutrient increase to a final level of 9.8 x 105 colony-forming units (CFU) ml-1. The increase was less pronounced in all greenwater qualities, which had 1-6 times lower bacterial numbers (Fig. 2).
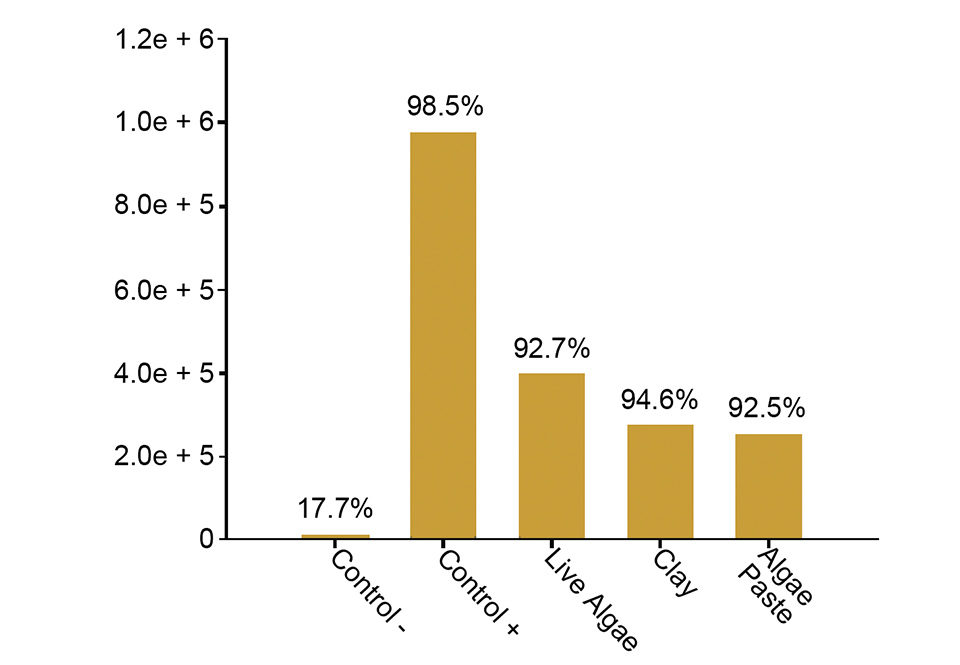
Live algae-associated bacteria seemed to contribute to the bacterial load, but on a moderate level. The nutrient up-shift stimulated blooms of opportunistic bacteria in all water treatments, but the use of greenwater techniques seemed to slightly delay their proliferation (Fig. 2). The Vibrio species fraction of the total bacterial community remained at a level below 3 percent, except for the two clearwater qualities, which both had a peak of 11 percent two to six hours after the up-shift.
(Editor’s Note: This article was originally published in the July/August 2007 print edition of the Global Aquaculture Advocate.)
Now that you've reached the end of the article ...
… please consider supporting GSA’s mission to advance responsible seafood practices through education, advocacy and third-party assurances. The Advocate aims to document the evolution of responsible seafood practices and share the expansive knowledge of our vast network of contributors.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year.
Not a GSA member? Join us.
Authors
-
K. Attramadal
Norwegian University of Science and Technology
NO-7491 Trondheim, Norway -
Y. Olsen
Norwegian University of Science and Technology
NO-7491 Trondheim, Norway -
O. Vadstein
Norwegian University of Science and Technology
NO-7491 Trondheim, Norway -
I. Salvesen
Norwegian Biodiversity Information Centre
Trondheim, Norway
Related Posts

Responsibility
Calcium and magnesium use in aquaculture
Aquatic plants and animals get the essential nutrients calcium and magnesium from water and food. Calcium concentrations impact the hydration and development of eggs in a hatchery, where calcium carbonate precipitation can be troublesome.

Responsibility
Erosion, sedimentation in earthen aquaculture ponds
The control of external inputs of suspended soil particles to ponds and internal erosion of embankments and bottoms should begin at the design and construction stage.

Responsibility
Examining copper use in aquaculture
Copper is used for control of the blue-green algae responsible for off-flavors in aquaculture animals, treating diseases and parasites, and avoiding cage net fouling. Although copper is an essential nutrient for plants and animals, an excess can negatively affect the environment and human health.

Responsibility
Liming materials for aquaculture
Liming materials neutralize acidity and increase pH in pond bottom soil and water. They also react with carbon dioxide to form bicarbonate and release calcium and magnesium, increasing both alkalinity and hardness concentrations in water.



