Unhampered by science, E.U. policies are too informal

detection of the broad-spectrum antibiotic chloramphenicol in shrimp imported to Europe from Asian countries brought about a flood of reactions within the European Community. They involved, among other actions, closing down European borders to seafood products (mainly shrimp) and making laboratories work overtime to analyze numerous batches of imported goods. The Netherlands went so far as to have seafood products containing the antibiotic destroyed, as they were regarded as a health hazard.
The EC tries to uphold a high level of food standards to ensure public health and safety. To this end, a precautionary approach – e.g., in the form of zero-tolerance standards for banned products – is used. The detection of trace amounts of chloramphenicol in shrimp and ensuing conflicts between the EC and exporting countries is an example of the effects of precautionary regulations.
Chloramphenicol banned
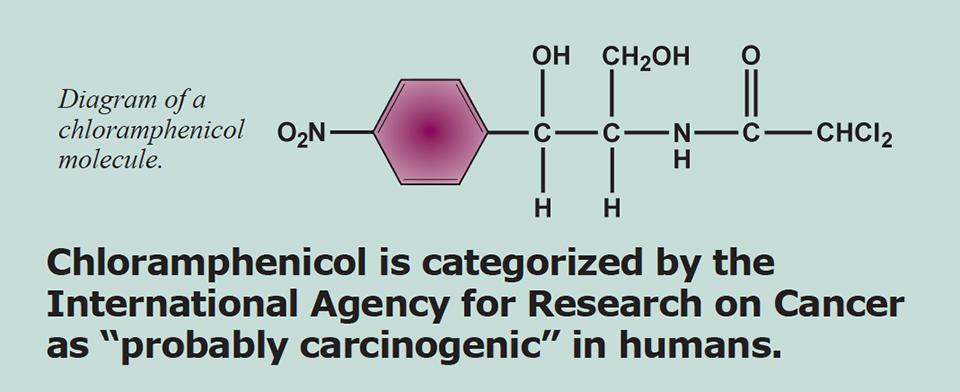
Chloramphenicol was placed on Annex IV of the Council Regulation EEC No. 2377/90 and is therefore not allowed in the production of food. The drug is categorized by the International Agency for Research on Cancer as “probably carcinogenic” in humans. The presence of chloramphenicol in food products is a violation of European law, as it is related solely to the illicit use of chloramphenicol in the food production chain. This translates into the legal, political, and public notion that detection of chloramphenicol – regardless of the concentration levels – poses a human health risk.
Risk assessment
Before discussing the implications of the precautionary approach of chemical food safety, the assessment of the risk to consumers of chloramphenicol in shrimp needs to be discussed. Concentrations of chloramphenicol found in shrimp vary roughly 1-10 ppb, or 1-10 micrograms per kilogram (1-10 nanogram per gram). One needs to know whether such concentrations indeed constitute a health risk to consumers. For that reason, the Dutch government asked the Dutch Institute for Environment and Public Health to assess the risk.
In the Netherlands, it was estimated that consumers eat an average of 4.0 grams of shrimp weekly. Consumers who eat fish and fish products an average of twice a week consume 8.4 g of shrimp. Using the highest levels of detected chloramphenicol (10 micrograms per kilogram) together with the higher intake of shrimp per week, the weekly intake of chloramphenicol comes to 84 tangrams per person per week, equaling 0.17 nanogram per kilogram body weight for a person weighing 70 kg. This could be regarded as a “reasonable” worst-case estimate of shrimp consumption.
Based on available toxicological data of chloramphenicol in relation to a maximum tolerable risk (MTR) level of 1:10 added cancer risk in the human population, the maximum allowed chloramphenicol intake was estimated to be 1-5 micrograms per kilogram body weight. The “reasonable” worst-case estimate is at least 5,000 times lower than the MTR level. Average exposure levels will, however, almost certainly be much lower. Therefore, the concentrations found in shrimp can be regarded as toxicologically irrelevant.
Food safety
In the public’s and politicians’ eyes, such man-made chemicals as pesticide residues, antibiotics, hormones, and the like are thought to be of central importance when discussing food safety and human health (Table 1). As shown by the chloramphenicol case, political institutions respond accordingly.
Hanekamp, Food safety ranking, Table 1
| 1. Food additives and contaminants (e.g., pesticide residues, antibiotics) 2. Environmental contamination (e.g., dioxins, PCBs) 3. Unbalanced diet (too much, too fat, one-sided) 4. Natural toxins (e.g., aflatoxins) 5. Microbial contamination (e.g., Salmonella typhimurium) |
|---|
1. Food additives and contaminants (e.g., pesticide residues, antibiotics) 2. Environmental contamination (e.g., dioxins, PCBs) 3. Unbalanced diet (too much, too fat, one-sided) 4. Natural toxins (e.g., aflatoxins) 5. Microbial contamination (e.g., Salmonella typhimurium) |
|---|
| No data available in table |
Table 1. Food safety ranking according to the general public.
However, much is scientifically known about the risks of food consumption. It is interesting to compare the public ranking of food product risks with the ranking derived from scientific knowledge (Table 2).
Hanekamp, Food safety ranking, Table 2
| 1. Unbalanced diet (too much, too fat, one-sided) 2. Microbial contamination (e.g., Salmonella typhimurium) 3. Natural toxins (e.g., aflatoxins) 4. Environmental contamination (e.g., dioxins, PCBs) 5. Food additives and contaminants (e.g., pesticide residues, antibiotics) |
|---|
1. Unbalanced diet (too much, too fat, one-sided) 2. Microbial contamination (e.g., Salmonella typhimurium) 3. Natural toxins (e.g., aflatoxins) 4. Environmental contamination (e.g., dioxins, PCBs) 5. Food additives and contaminants (e.g., pesticide residues, antibiotics) |
|---|
| No data available in table |
Table 2. Food safety ranking according to science.
This ranking makes it clear that chemical food safety is not of prime concern when dealing with food consumption and human health. This, however, goes against the grain of so-called public and political “wisdom.” It is highly politically incorrect to question the exuberant regulation of pesticide, antibiotic, and hormone resi-dues in relation to human health.
Relative risk
Consider, however, risk ranking of various food issues from a somewhat different perspective. Table 3 ranks relative risk on a scale of 1 to 100,000.
Hanekamp, Relative ranking of food safety, Table 3
| Food Issue Ranking | Relative Importance |
|---|
Food Issue Ranking | Relative Importance |
|---|---|
| Microbial contamination | 100,000 |
| Nutritional imbalance | 100,000 |
| Environmental contamination | 100 |
| Natural toxins | 100 |
| Pesticide residues | 1 |
| Food additives | 1 |
Human health and food consumption are essentially related to microorganisms and nutritional imbalance. Man-made chemicals are “not on the list.” Why, then is food regulation so concerned with chemical food safety; and why are public and political concern, translated or initiated by nongovernmental organizations, focused primarily on the chemical aspects of food? The rise of the “cautious culture” is directly related to this.
Cautious culture
In his 1986 paper, “Risikogesell-schaft: Auf dem Weg in eine andere Moderne,” Ulrich Beck coined the concept of the “risk society,” which now is common currency even outside the field of social scientists. Beck’s basic idea was that industrial society has developed to such an extent (in the First World) that the distribution of scarce goods is no longer the primary social problem. The main problem, Beck claimed, is the distribution of the technological risks that are also a product of industrial production, as is the commercial exploitation of scientific knowledge.
Some 16 years later, there is little doubt that Beck and others with similar ideas came up with some very insightful observations and predictions. Major problems in today’s Western society center on safety and security. Major worries focus not so much on our wealth, but our health.
Future thought
This opening up of the future as something to think – and worry – about today has contributed to changes in our way of thinking about risks to our health and the environment. My contention is that as the risk society develops, we shall see the development of a new culture. If industrial society knew a risk culture, then risk society will have a cautious culture. The rise of the precautionary principle (PP) in past decades in the Western World is precisely in line with this contention.
Risk shift
Risks in the modern-day societal focal point are related to human economic and industrial activities. The paradox is that growing wealth as a result of increasing economic activities results in awareness of the risks spawned by this same economic evolution. Risks related to the “natural background” of human existence – influenza, insect bites, drowning, microbial food poisoning, and so on – are more or less not included in this risk portfolio.
In other words, the post-modern public and political risk portfolio is mainly related to economic activities, called in general market failure. This perceived failure is presently countered by a tradition of precautionary regulation regarded as devoid of policy failure, an exogenous panacea.
Precautionary principle

Precautionary regulation has a long history in both Europe and the United States, coinciding with the development of the risk society. Prominent endorsements have appeared on both sides of the Atlantic since at least the 1970s. The cognate concept of “vorsorgeprinzip” in German law dates to the early 1970s.
However, precaution in the form of the precautionary principle has lately become a centralized theme within environmental issues, especially when scientific knowledge concerning a specific environmental or human health risk is wanting or even lacking. It is paid homage in several important international agreements. The treaty that constitutes the European Union expressly provides that E.U. policy on the environment “shall be based on the precautionary principle”.
London declaration
In the 1987 London Declaration about the protection of the North Sea, the PP is formulated as follows:
“In order to protect the North Sea from possibly damaging effects of the most dangerous substances, a precautionary approach is necessary which may require action to control inputs of such substances even before a causal link has been established by absolute clear scientific evidence.”
The PP seeks to advance the timing and tighten the stringency of regulation. On sliding-scale dimensions, regulation is “more precautionary” when it intervenes earlier and/or more stringently to prevent uncertain future adverse consequences.
U.S. regulation
Despite its adoption of a “precautionary preference” in some statutes, the United States has not officially adopted the PP as a general basis for regulation. After endorsements of precautionary regulation in cases like Ethyl Corp. versus EPA, and TVA versus Hill in the 1970s, the U.S. Supreme Court ruled in the Benzene case (Industrial Union, AFL-CIO versus API, 1980) that the Occupational Safety and Health Administration cannot implement regulation on the basis of speculation about uncertain risks.
This decision and a 1983 guidebook from the National Academy of Sciences spurred widespread adoption of risk assessment as the basis for U.S. risk regulation. Along these lines, the U.S. has resisted all-inclusive statements of the PP. For example, the U.S. insisted on qualifying the statement of the PP in the Climate Change Convention.
Contrasting assessment
A common deduction is that Europe underwrites the PP and proactively seeks to regulate risks, while the U.S. opposes the PP and waits more circumspectly for evidence of actual harm before regulating. In other words, U.S. regulation is more rooted in the science of formally assessing risks before acting, whereas E.U. regulation is more qualitative and puts forward policies through informal decision making more or less unhampered by science.
These juxtapositions partly explain the eagerness among advocates of the PP to have it made part of customary international law so the United States, among others, cannot resist it. Some paint the picture of a civilized, safe Europe contrasting a “dangerous,” “risk-taking” America.
In his 2001 paper, “Precaution in a Multi-Risk World,” J. B. Wiener shows this inference is erroneous, both descriptively and normatively. In his words:
“In a world of multiple risks, the reality is more complicated. First, although precaution can be warranted, it is not universally desirable. Sometimes it is for the best, but sometimes precautionary regulation is too costly, and sometimes – given multiple risks – precaution can even yield a perverse net increase in overall risk. Context is crucial.”
The issue of context – relating chemical food safety to the other food safety issues at hand – has been lost completely when reviewing the chloramphenicol case.
Critique of cautious regulation
Food safety regulations are especially focused on chemicals related to human interventions in the food production chain (e.g., dioxins, PCBs, anti-biotics, pesticides, prions (BSE), and others). Zero tolerance toward chemicals is the ultimate consequence of this, although, as shown above, chemical food safety is not a real issue in the ranking of food-related risks.
In the case of chloramphenicol, this translates into the practice of finding the best analytical techniques to determine the lowest concentrations of this illicit compound. The achievement of ever-lower limits of detection has become a goal in itself, as the legal perspective is transparent and widely publicized: no single molecule is allowed.
This cautious approach, embodied in the PP, is counterproductive on multiple levels. It is not focused on food safety, but maintaining food-safety regulations. Several risk tradeoffs or countervailing risks are the results of precautionary food safety regulations.
Zero tolerance vs. toxicological relevance
A zero-tolerance approach – in practice the limit of detection (LOD) – for certain chemicals in food is not related to toxicological relevance per se. With the advent of new and more advanced analytical equipment, the ensuing lower LOD will increase the chance of finding certain chemicals in food. Environmental and ecological background concentrations of all kinds of chemicals – including antibiotics – come in vogue with ever-lower LOD.
The safety of food seems, therefore, appears to decrease over time as more chemical compounds are detected. However, detecting more compounds at increasingly lower levels reflects only advancing technological capabilities, not deteriorating food safety.

Risk tradeoffs
Tradeoffs are the inevitable consequences of the present, cautious mono-thematic approach of food safety. By scrutinizing the chemical safety of food products, other aspects of food safety run the risk of receiving a lower priority in funding and research, as well as public and political attention.
Moreover, such an approach to food safety holds the risk of intensifying the search for banned chemicals, which would tie up budgets, research efforts, and personnel to the detriment of food safety as a whole. H. Sapolsky (“The Politics of Risk”) puts it as follows:
“Government policies add to the confusion of risk. There are contradictory statements about particular risks and inconsistent rankings among them. … That policies are contradictory within and between jurisdictions, and that they may change as does the calendar, is due to the structure of government and the fact that the agencies are subject to political masters who must respond to public pressures in order to retain office.
“Convinced that they must appear willing to alleviate every product or environmental fear as it arises, officials make no effort to pursue consistent, carefully designed policies toward health risks. Whatever the scare of the day, officials stand ready to formulate quickly congressional testimony, briefing papers, news releases, and programs that demonstrate their unsurpassed commitment to protecting the public.”
Media attention, political importance, and current scientific funding can result in the over-exaggeration of food safety risks. It is therefore not surprising that different policies concerned with one issue (e.g., public health) differ wildly in cost effectiveness.
Another risk tradeoff of cautious food safety management is the export of socioeconomic and public health risks from the E.U. to countries pres-ently not able to meet European standards. This could result in a step toward higher standards within exporting countries. More realistically, though, it could result in unemployment within food-production industries in those exporting countries, due to lack of funding to realize the higher standards.
Precaution vs. risk management
Precaution makes risk management superfluous, as the PP encourages a partial, asymmetric view of reality by focusing only on certain risks one wants to avoid. Therefore, it promotes irrational behavior by the assumption that the costs of avoidance can be met on any scale. The notion of limitless funding is closely tied to the implementation of the PP.
The E.C. states that invoking the PP is a political decision about acceptable risk in light of the high level of protection deemed necessary. However, when discussing the necessary proportionality of measures, this proportionality does not refer to the risk but to the chosen level of protection. Therefore, a ban or zero-tolerance approach is considered the appropriate measure for chloramphenicol, despite the fact that the “reasonable” worst-case estimate of its exposure is at least 5,000 times lower than the MTR level.
Risks Vs. benefits
A cautious policy focus on certain chemical risks of food might make the general public shy away from certain beneficial food products to the overall detriment of public health. The human health risks as a result of lower seafood consumption – because of a negative public attitude – could well overshadow the very low potential risk of chloramphenicol exposure avoided.
Conclusion
Food safety is related to the overall toxicological and microbiological profile of food products. Intake of food has both benefits and risks on the basis of that same profile. Products presented to the public should be “safe” from a toxicological and microbiological perspective. Risk management of food safety is in need of a worst-things-first approach from a financial, economic, political, and scientific perspective, as all these aspects are in some way limited. To be sure, banned products should not be present. However, toxicological relevance should be the driving force for policy making, as advancing technology will result in lower levels of detection.
Choosing a precautionary zero-tolerance approach toward banned chemicals makes for irresponsible government. Any amount of a banned chemical in food products becomes a case for criminal investigation. Food safety is less at stake than upholding the law. This approach paradoxically combines a profound pessimistic view of the global economy with a naïve optimism of policy intervention capabilities and efficiency.
A food safety utopia is envisioned when the precautionary principle is implemented. But the PP encourages a very partial, asymmetric view of reality by focusing only on the risks one wants to avoid, and remaining oblivious of the risks potentially introduced by the policy itself. Moreover, the asymmetry is increased by the fact that the policy makers who invoke the PP do not need to adhere to it themselves. Indeed, precaution empowers bureaucracy, as the PP (when applied fully and logically) would cannibalize itself and potentially obliterate all cautious regulation.
The PP speaks as though government regulation were a panacea for environmental and social ills. In other words, market risks warrant governmental regulation. But government regulation is not an exogenous solution to social problems. It is itself an endogenous and fallible human activity, and as such can create risks as real as the risks of the market activities.
International social and economic tensions and political struggles on food safety issues do not revolve around the toxicological profile of the food products in question, but on the liability of those responsible for the presence of trace amounts of banned chemicals. If this trend continues, it will have serious global economic, social, and political repercussions.
The chloramphenicol issue is a primary case in hand. The European society could become increasingly risk-averse and economically stagnant, addressing only the perception of food safety and leaving the real food safety issues out of focus to the detriment of public health and economic progress in exporting countries.
U.S. Information Quality Act: A way out?
As shown in this article, the European cautious approach is not the way to go when discussing the breadth and the depth of food safety. In the United States, in my opinion, a way foreword has been found in the form of the Information Quality Act (IQA). Within this framework, the Office of Management and Budget has issued guidelines for ensuring and maximizing the quality, objectivity, utility, and integrity of information disseminated by federal agencies.
This requires federal agencies to make available on their websites guidelines in accordance with OMD standards, including administrative mechanisms that allow affected persons to seek and obtain correction of information maintained and disseminated by the agency that does not comply with the guidelines.
Improved risk management
A number of important improvements on current cautious risk management strategies have been made within the food production sector. First of all, the IQA urges administrations to strive for maximimum scientific input. Moreover, the possibility of appeal by affected stakeholders is implemented within the IQA.
A higher awareness of risk tradeoffs resulting from single-minded policies such as in the chloramphenicol issue is to be expected. Prevention of the abuse of cautious risk management could keep issues out of legal courts and result in depoliticization of essentially technical issues.
Of course, whether such a bright future is indeed in store remains to be seen. However, a recent judgment of the European Court on antibiotic feed additives – despite its adherence to the PP and its ensuing affirmation of the ban on such products – clearly stated:
“A preventive measure cannot be founded on mere conjecture which has not been scientifically verified, but may be taken only where there is a real risk. In the court’s view, the concept of risk entails some probability that the negative effects which the measure is specifically designed to prevent will occur. The degree of risk cannot be set at ‘zero risk.’”
This might be a first step in a rationalization process severely needed in discussions on food safety.
A preventive measure cannot be founded on mere conjecture which has not been scientifically verified, but may be taken only where there is a real risk. – European Court
HAN Foundation…
Heidelberg Appeal Nederland (HAN) is an independent, nonprofit foundation of scientists and science supporters whose aim is for science and realistic risk analyses to play a greater role in public opinion and policy decisions. Its primary roles are to contribute to scientific debate; provide an independent voice on environmental and biotechnological issues to the media, general public, and educators; and ultimately provide balance on scientific issues.
Established in the Netherlands in 1993, HAN was named after the Heidelberg Appeal, a declaration signed in 1992 by over 3,500 scientists. Members are accepted from all walks of life and all branches of science. HAN currently has over 800 donors, including almost 200 professors.
One of the activities of Heidelberg Appeal Nederland is scientific research conducted at the request of third parties. Such research is performed by the HAN Foundation only, supported by an independent scientific supervisory committee. To ensure their studies are executed in an independent fashion, HAN retains the right to publish results regardless of the outcome of the research.
Note: For more information, see the author’s 2002 HAN paper “From Cautious to Risk Management of Chloramphenicol in Shrimp: An Introductory Food-Safety Position Paper.”
(Editor’s Note: This article was originally published in the October 2002 print edition of the Global Aquaculture Advocate.)
Now that you've reached the end of the article ...
… please consider supporting GSA’s mission to advance responsible seafood practices through education, advocacy and third-party assurances. The Advocate aims to document the evolution of responsible seafood practices and share the expansive knowledge of our vast network of contributors.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year.
Not a GSA member? Join us.
Author
-
J.C. Hanekamp, Ph.D.
Chief Executive Officer
Heidelberg Appeal Nederland Foundation
P.O. Box 75311
1070 AM Amsterdam
Tagged With
Related Posts

Innovation & Investment
American Unagi brings eel farming back ‘home’
Sara Rademaker launched American Unagi to shift eel farming to American soil, where the eels are from. Why? Because of the novelty, and because she saw an opportunity to do things better.

Health & Welfare
Antibiotic-resistant bacteria, part 1
No antimicrobial agent has been developed specifically for aquaculture applications. However, some antibiotic products used to treat humans or land-based animals have been approved for use at aquaculture facilities.

Health & Welfare
Antibiotics in aquaculture: Is responsible use possible?
Regulations on antibiotics in aquaculture vary by country and region, from outright bans to minimal oversight.

Intelligence
Expanding tilapia supply meets growing global market
Global tilapia production and consumption growth has mirrored the species' versatility. The fish will remain integral to rural aquaculture development.



