Outbreak forced production shift from intensive to semi-intensive

In 2003, the infectious myonecrosis virus (IMNV) severely disrupted the production of farm-raised shrimp in Brazil after five years of continuous growth that had led to record production. The virus was first detected in September 2002 in farms located in the state of Piauí and rapidly spread to neighboring states within six months. Since then, production has recovered mainly by shifting from intensive to semi-intensive production methods.
Symptoms, treatment
Shrimp affected with IMNV typically show a loss of transparency in the abdominal tissue that often leads to a reddish color and persistent daily mortality after shrimp reach 7 g in body weight. Cumulative mortalities can approach 70 percent over a typical 120-day growout cycle and result in feed-conversion ratios well above 2 for 12- to 14-g shrimp.
To date, no single effective treatment against shrimp viral diseases exists. As shrimp are not able to respond to vaccination, enhancement of disease resistance has been attempted through stimulation of their immune systems. Several compounds have been reported to actively promote immune responses and increase survival in cultured penaeids after viral or bacterial challenge. These include peptidoglycans, glucans, lipopolysaccharides and sulfated fucoidans.
Brazil study
In a recent study funded by a grant from Financiadora de Estudos e Projetos, the authors’ goal was to determine if a beta-1,3/1,6-glucan extracted from the cellular wall of the bakers yeast Saccharomyces cerevisiae could promote increases in the survival and growth of Pacific white shrimp (Litopenaeus vannamei) after an oral challenge with IMNV.
Shrimp growth and survival of juvenile L. vannamei orally challenged with IMNV were evaluated in 20 circular, 500-l polypropylene tanks stocked with 57 shrimp/tank (100 shrimp per square meter). Shrimp were fed for 70 days with lab-manufactured diets either deprived or containing a source of the beta-glucan.
The experimental design comprised one treatment and three control diets (Table 1): COM, a high-performance commercial diet fed to IMNV-free shrimp; REF, a basal diet manufactured in the lab without the beta-glucan and fed to IMNV-free shrimp; IREF, the basal diet fed to shrimp orally challenged with IMNV; and IBET, the basal diet with an inclusion of 1,000 mg/kg of a commercial beta-1,3/1,6-glucan.
Nunes, Experimental design for shrimp challenged, Table 1
| Treatment | Oral IMNV Challenge | Beta-1, 3/1,6-glucan Supplementation |
|---|
Treatment | Oral IMNV Challenge | Beta-1, 3/1,6-glucan Supplementation |
|---|---|---|
| COM (35.0% crude protein) | No | – |
| REF | No* | No |
| (31.4% crude protein) | ||
| IREF | Yes | No |
| IBET | Yes | 1,000 mg/kg |
The nearly isoenergetic and isoproteic diets were designed to result in the same nutritional composition except with regard to the inclusion of a beta-glucan source. The beta-glucan was included in the IBET diet at the expense of bentonite (Table 2). Over the growth cycle, shrimp were exposed to the feed for five hours daily. Meals were split equally between the two morning and evening feeding times.
Nunes, Composition and chemical proximate values of diets, Table 2
| Ingredients | Feed (g/kg of diet) REF, IREF | Feed (g/kg of diet) IBET | Feed (g/kg of diet) COM |
|---|
Ingredients | Feed (g/kg of diet) REF, IREF | Feed (g/kg of diet) IBET | Feed (g/kg of diet) COM |
|---|---|---|---|
| Wheat flour | 350.0 | 350.0 | – |
| Soybean meal | 220.8 | 220.8 | – |
| Broken rice | 70.0 | 70.0 | – |
| Fishmeal, anchovy | 167.1 | 167.1 | – |
| Fishmeal, offal and by-catch | 100.0 | 100.0 | – |
| Fish oil | 30.0 | 30.0 | – |
| Soy lecithin | 20.0 | 20.0 | – |
| Cholesterol | 1.5 | 1.5 | – |
| Attractant | 5.0 | 5.0 | – |
| Common salt | 10.0 | 10.0 | – |
| Vitamin-mineral premix | 10.0 | 10.0 | – |
| Synthetic binder | 5.0 | 5.0 | – |
| Bicalcium phosphate | 12.0 | 12.0 | – |
| Betonite | 3.6 | 2.6 | – |
| Beta-1,3/1,6-glucan | 0 | 1.0 | – |
| Chemical Composition | |||
| Crude protein (%, dry matter) | 31.4 | 31.3 | 36.6 |
| Crude fat (%, dry matter) | 10.1 | 10.0 | 8.7 |
| Ash (%, dry matter) | 10.0 | 10.3 | 10.6 |
| Crude fiber (%, dry matter) | 5.1 | 4.3 | 7.6 |
| Gross energy (kcal/kg) | 3,706 | 3,817 | 3,713 |
IMNV challenge
Shrimp suspected of IMNV infection were collected in the geographic area where the first case of the disease was reported in Brazil. Sampled shrimp were 12 to 14 grams in body weight and exhibited severe gross signs of IMNV infection. Additionally, shrimp in the growout pond surveyed had attained a low 32 percent final survival at harvest.
Soon after capture, shrimp pleopods from 50 animals were fixed in a 95 percent ethanol solution for polymerase chain reaction (PCR) and real-time PCR analyses. For assurance, the size of the pleopod sample exceeded the amount required for analysis. The shrimp samples were found negative for Taura syndrome virus, white spot syndrome virus and infectious hypodermal and hematopoietic necrosis virus , but positive for IMNV.
In the lab, IMNV-free shrimp were fed roughly ground IMNV-contaminated tissue. Challenge occurred over three consecutive days, when shrimp had reached 4.9 to 6.9 grams in body weight or 29 days after continuous exposure to the experimental diets. Feeding rates over the challenge period varied 4.0 to 5.2 percent of estimated stocked shrimp biomass. During the viral challenge period, shrimp in treatments COM and REF were fed their regular diets.
Results
Shrimp survival started to decrease progressively after the oral challenge (Fig. 1). Three days after exposure to IMNV extract, shrimp mortality in the IREF treatment was already statistically different from all other treatments (P < 0.05). This trend prevailed until shrimp harvest. Conversely, cumulative shrimp mortality did not vary significantly among the COM, REF and IBET treatments (P > 0.05).
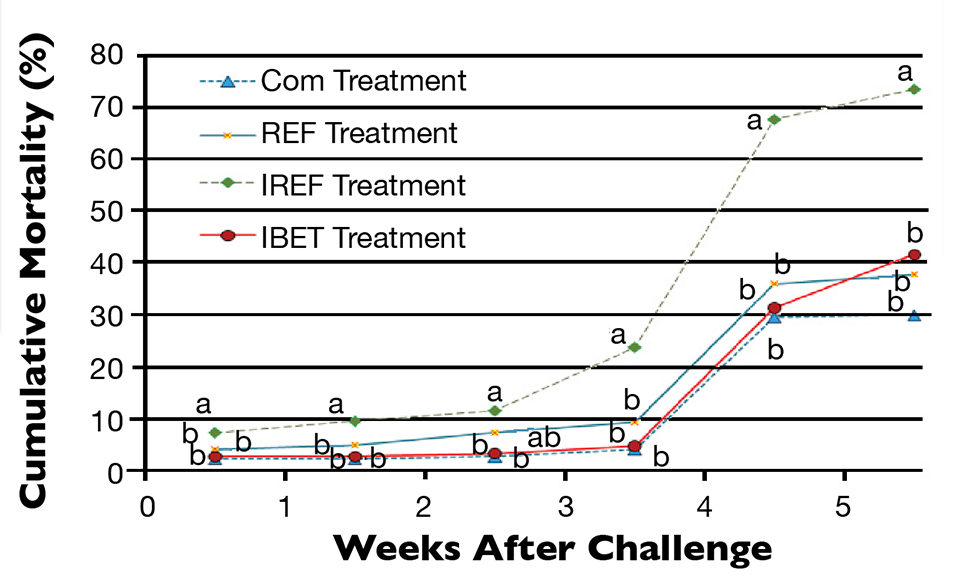
Mortality of 30 percent and greater was observed three weeks after the viral challenge, regardless of the feed treatment. However, in the IREF treatment, shrimp mortality topped 67 percent.
At harvest, the highest shrimp survival rate (69.5 ± 12.7 percent) was observed in the COM treatment (Fig. 2), which did not differ statistically from the REF group. In contrast, the poorest survival (23.20 ± 5.76 percent) was reported for shrimp in the IREF treatment. Shrimp survival for IBET (48.10 ± 8.53 percent) was significantly higher than in IREF.
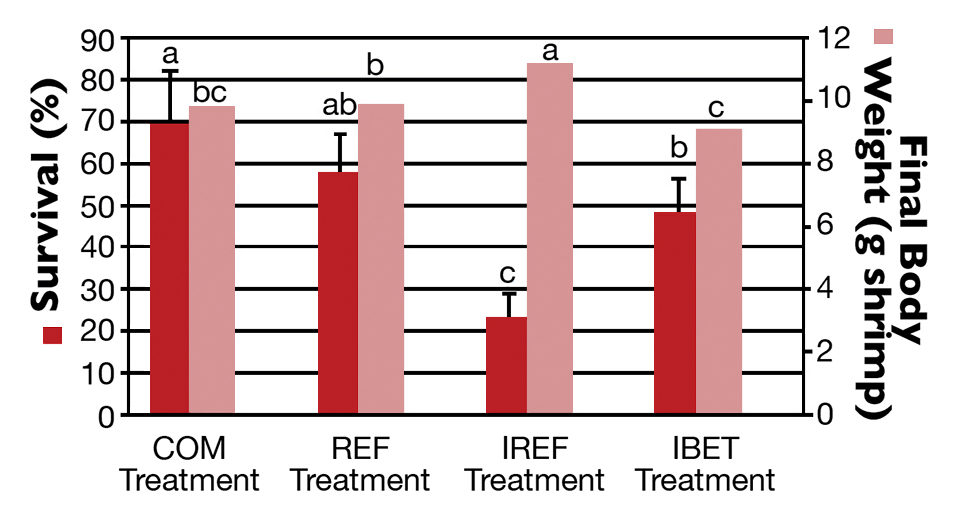
Shrimp grew continuously over the rearing period. Weekly growth rates varied from 0.56 grams for the first 14 days of rearing to 0.77 grams two weeks prior to harvest. At harvest, the highest shrimp body weight (11.20 ± 0.58 grams per shrimp) corresponded to the lowest survival rate (IMNV-Ref; Fig. 2).
Shrimp body weight in the IREF treatment was significantly higher than in the other treatments (P < 0.05). The final weights for COM and REF shrimp were not statistically different, while shrimp final weight for IBET was statistically lower than for the REF group (P < 0.05).
Observations
In the study, the IREF and IBET animals were found very susceptible to IMNV. In the former, average mortality as high as 76.8 percent was observed at harvest, when animals were close to 8 grams in body weight. This observation agrees with reports from shrimp farmers. In infected areas, clear signs of IMNV could be found in shrimp of all sizes, but the highest mortality rates were most often observed when animals were 6 to 8 grams in body weight.
The study results suggested that continuous exposure to a diet supplemented with beta-1,3/1,6-glucan enhanced L. vannamei survival after oral challenge with IMNV. However, the effects of the beta-glucan on shrimp growth performance were unclear, probably due to the significant mortalities observed.
(Editor’s Note: This article was originally published in the September/October 2010 print edition of the Global Aquaculture Advocate.)
Now that you've reached the end of the article ...
… please consider supporting GSA’s mission to advance responsible seafood practices through education, advocacy and third-party assurances. The Advocate aims to document the evolution of responsible seafood practices and share the expansive knowledge of our vast network of contributors.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year.
Not a GSA member? Join us.
Authors
-
Alberto J.P. Nunes, Ph.D.
Instituto de Ciências do Mar
Av. da Abolição, 3207 – Mereles
Fortaleza, Ceará 60165-081 Brazil -
Hassan Sabry-Neto, M.S.
Instituto de Ciências do Mar
Av. da Abolição, 3207 – Mereles
Fortaleza, Ceará 60165-081 Brazil -
Marcelo V. C. Sá, Ph.D.
Instituto de Ciências do Mar
Av. da Abolição, 3207 – Mereles
Fortaleza, Ceará 60165-081 Brazil
Tagged With
Related Posts

Aquafeeds
A look at corn distillers dried grains with solubles
Corn distillers dried grains with solubles are an economical source of energy, protein and digestible phosphorus to reduce feed costs and fishmeal usage.
Health & Welfare
Brazil shrimp farm performs genetic selection for IMNV resistance, growth
The Queiroz Galvão Alimentos shrimp farm and hatchery in Brazil have been working with Concepto Azul to implement a disease-prevention and genetic-breeding program that addresses ongoing impacts from infectious myonecrosis virus (IMNV) and other pathogens.

Innovation & Investment
Aquaculture planning, development in Brazilian federal waters
The aquaculture industry in Brazil is moving toward further expansion with the support of the federal government. A key strategy of the More Fishing and Aquaculture plan is the development of aquaculture in federal waters. The plan promotes sustainable development of fisheries and aquaculture by linking those involved and consolidating state policies addressing social inclusion, security and food sovereignty. Tilapia is the main farmed fish, although tambaqui and others have potential for large-scale production due to their wide acceptance by consumers.

Health & Welfare
Bacillus probiotics benefit tilapia rearing under challenging conditions in Brazil
A recent study that evaluated the benefits of using probiotics with a balanced mixture of Bacillus bacteria strains to inhibit pathogenic bacteria in tilapia.



