In vivo infection modeling analyzes the dynamics of horizontal transmission of White Spot Disease in L. vannamei and evaluates the importance of specific environmental components

For three decades, White Spot Disease (WSD) has been the most widespread and lethal disease in shrimp aquaculture worldwide. To develop and improve effective mitigation strategies, a better understanding of the dynamics of White Spot Syndrome Virus (WSSV) epidemics and transmission is needed but studies that have examined the dynamics of WSSV transmission in L. vannamei are scarce.
Viral diseases in aquaculture are the result of the interplay between pathogen, host, and environment. The transmission dynamics of a specific WSSV strain in an animal population depend on the virulence of that WSSV strain, the density of the susceptible hosts, the individual host defense status, and recovery rates. To accurately characterize these dynamics, however, it is necessary to first understand the time course of WSD in an individual host.
Three main routes of WSSV transmission have been reported: (1) a vertical transovarial transmission from broodstock to progeny; (2) horizontal transmission through ingestion of WSSV-infected carcasses; and (3) horizontal transmission by exposure to water-borne WSSV. According to some researchers, in L. vannamei, horizontal transmission through consumption of infected tissue, by cannibalism or predation, is often considered to be the most effective path of infection compared to exposure to water containing WSSV virions. But a more recent study contested these findings, suggesting that direct contact transmission, for which cannibalism was considered a co-factor, was of minor importance for L. vannamei compared to indirect environmental transmission. Therefore, the question was raised why the results from these studies were contradictory.
This article – summarized from the original publication (Cox, N. et al. 2023. The Way of Water: Unravelling White Spot Syndrome Virus (WSSV) Transmission Dynamics in Litopenaeus vannamei Shrimp. Viruses 2023, 15(9), 1824) – reports on a study that used in vivo infection modeling as a tool to analyze the dynamics of the horizontal transmission of WSD in L. vannamei, and to evaluate the importance of specific environmental components that might be involved in this process.
Study setup
Specific-pathogen-free (SPF) L. vannamei postlarvae were procured from Global Blue Technologies (Rockport, TX, USA). These shrimp were certified as SPF for WSSV and various other pathogens. At the IMAQUA, Aquaculture Immunology Technologies (Lochristi, Belgium), the animals were reared in a recirculating aquaculture system (RAS) equipped with 470-liter tanks with artificial seawater (salinity 20 ppt).
For the experiments, shrimp were randomly selected from the rearing tanks and transferred to the disease challenge facility, where they were stocked either in 10-liter or 290-liter challenge tanks and acclimatized for three days prior to the start of the trials. During the infection trials, shrimp were fed at a fixed feeding rate set at a level of 6.5 percent of their body weight.
First, we performed a natural history of disease study in an individual infection model using the WSSV Thai-1 strain, which has been extensively researched in L. vannamei. Second, we developed a reproducible experimental infection model in which shrimp were housed in groups. Third, this model was then used for an observational epidemiological study to identify the characteristics of an epidemic caused by Thai-1. Finally, we employed this model to investigate the role of specific environmental components (i.e., molts, feces, water) in WSSV transmission dynamics.
For detailed information on the experimental design and animal husbandry; WSSV challenge; data collection and analyses, refer to the original publication.
Results and discussion
In this study, the dynamics of WSSV epidemics and transmission were elucidated in controlled experimental infection models. An oral inoculation method earlier established for the WSSV Thai-1 strain was used to emulate natural WSSV infection in the field. The WSSV Thai-1 strain has been extensively examined in L. vannamei and is known to be highly virulent. However, a study to characterize the natural course of WSD caused by this strain via natural feeding on WSSV-infected tissues was never conducted.
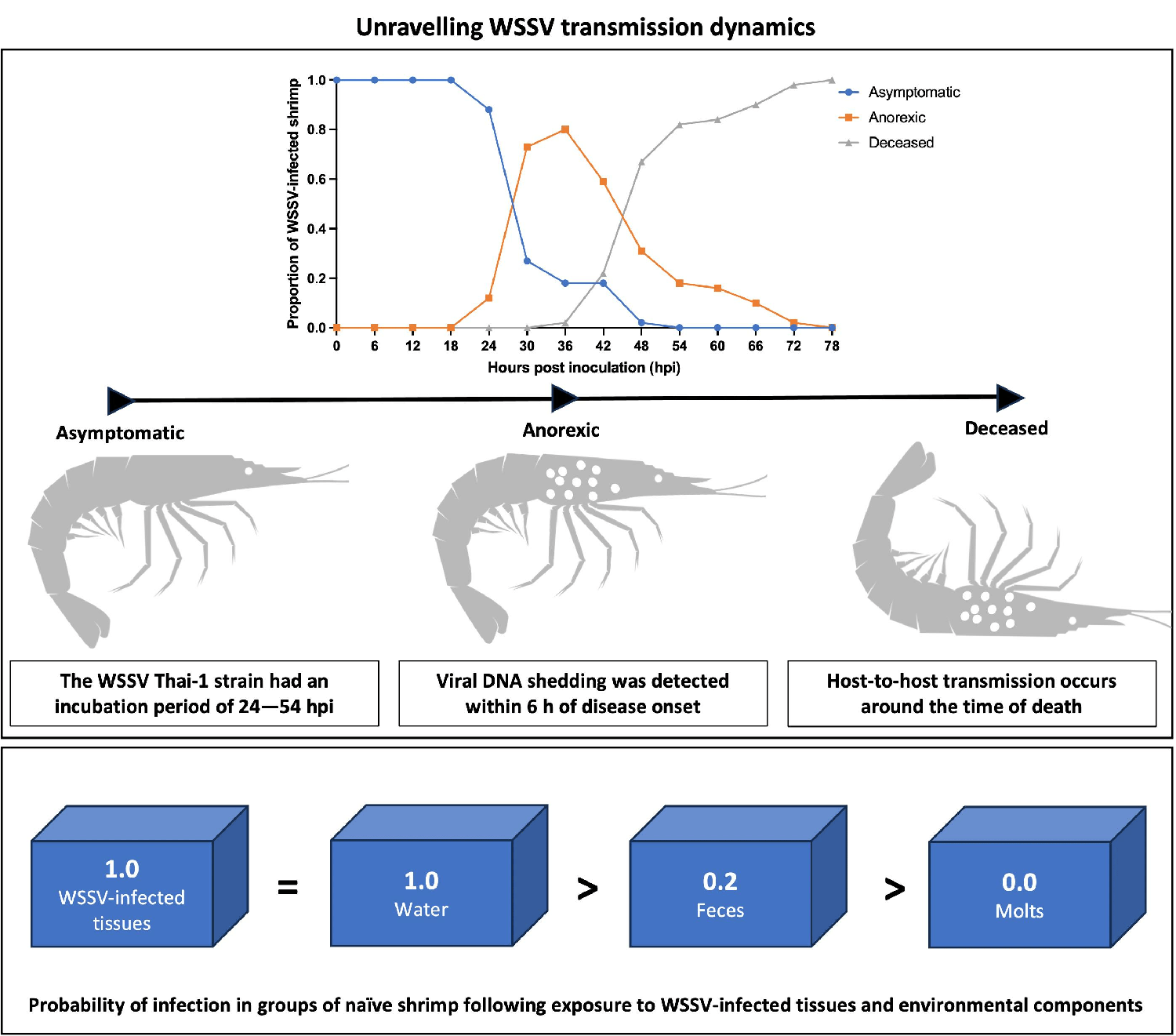
The first experiment of this study established the rapid time course of this disease with an onset of clinical symptoms at 24–30 hours post inoculation (hpi), and the probability of death already being at its highest between 42–48 hpi. Moreover, the disease irreversibly leads to death no later than 78 hpi. These findings were imperative to the accurate analysis of the epidemic patterns of spread in our third experiment. Furthermore, these observations imply that a time-based intervention in the event of an outbreak caused by a virulent WSSV, such as WSSV Thai-1, would be the most appropriate, and this must be considered when developing countermeasures.
During the first experiment, most WSSV-infected animals started shedding viral DNA in the water within 6 hours of disease onset. Additionally, our data indicated that viral DNA shedding intensified over the course of the disease, so that the WSSV DNA concentration in the tank water reached its peak around the time of death. This suggests that there was a correlation between WSD severity and the viral DNA shedding rate of infected shrimp, which is supported by another study that was recently published. It should be noted, however, that using PCR methods does not give information about the detected virus’s ability to infect a susceptible host. Thus, direct conclusions between WSD severity and the shedding of infectious virions cannot be made.
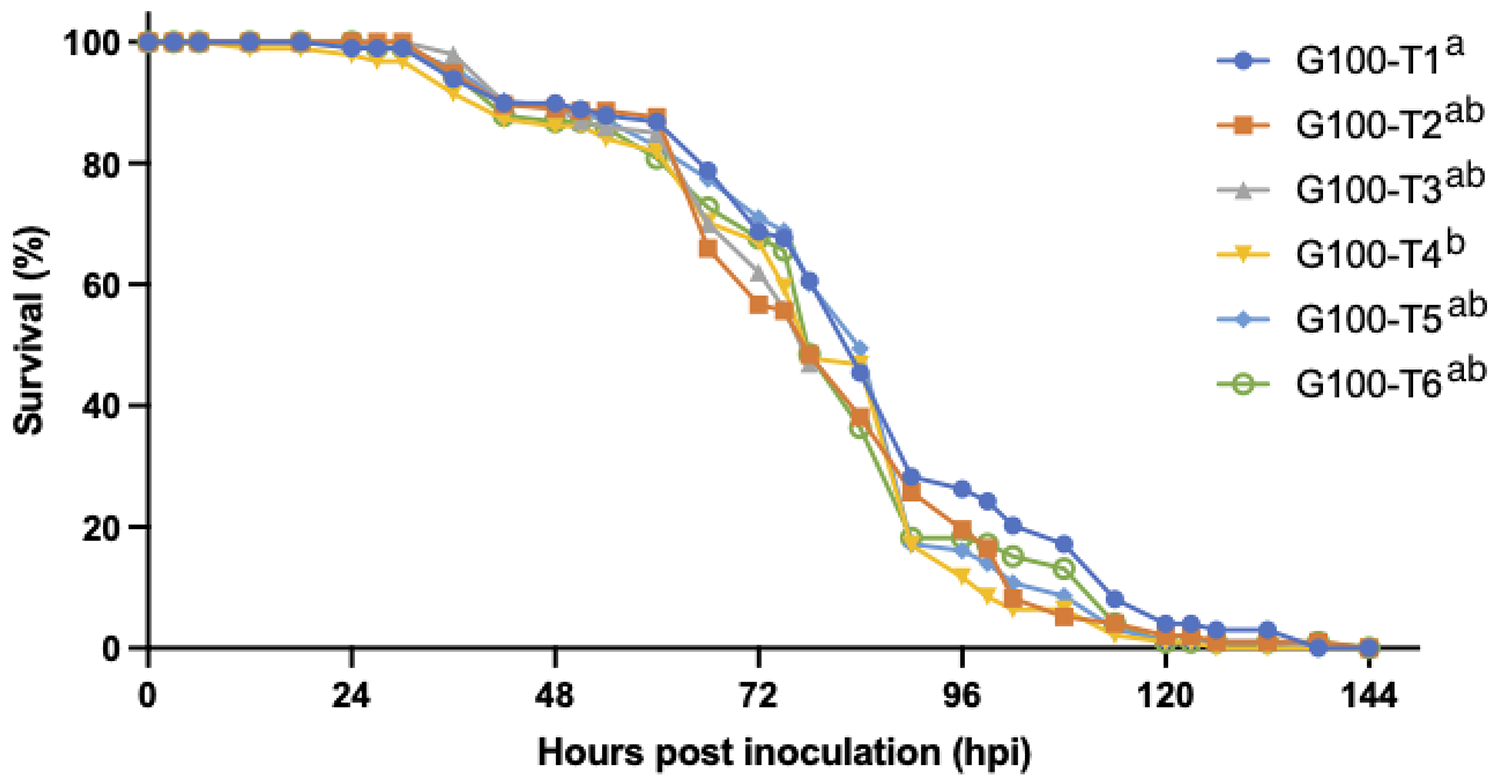
High densities have been identified as an important risk factor for WSD. To develop a reproducible experimental group infection model that would be suited to use for epidemiological studies, we aimed to find a threshold density at which a WSD epidemic would always occur. Indeed, when increasing the experimental population density from 2 individuals per 10 L to 10 individuals per 10 L in our second experiment, the risk of initial infection in a tank after exposure to WSSV-infected tissues increased significantly. However, once WSD manifested in at least one shrimp in a tank, all shrimp in that 10-L tank were invariably lethally infected during the experiment, regardless of the stocking density.
The effect of isolation on WSSV epidemic and transmission dynamics was studied in our third experiment. The results show that the isolation of the animals acted as an adequate control measure to reduce the size of the WSD epidemic in this experimental setting. The earlier this measure was taken, the more effective it was to decrease the number of secondary cases and to prevent a fully developed outbreak. Indeed, this indicates again that a time-based intervention in case of an outbreak in the field, for instance by administration of effective prophylactic treatments, might still prevent mass mortalities. These findings also demonstrate that the first cases of host-to-host transmission start occurring between 30 and 48 hours after inoculation. Additionally, the third experiment confirmed that sentinel populations could be infected by housing them in the vacant tanks that had been previously occupied by an infected population.
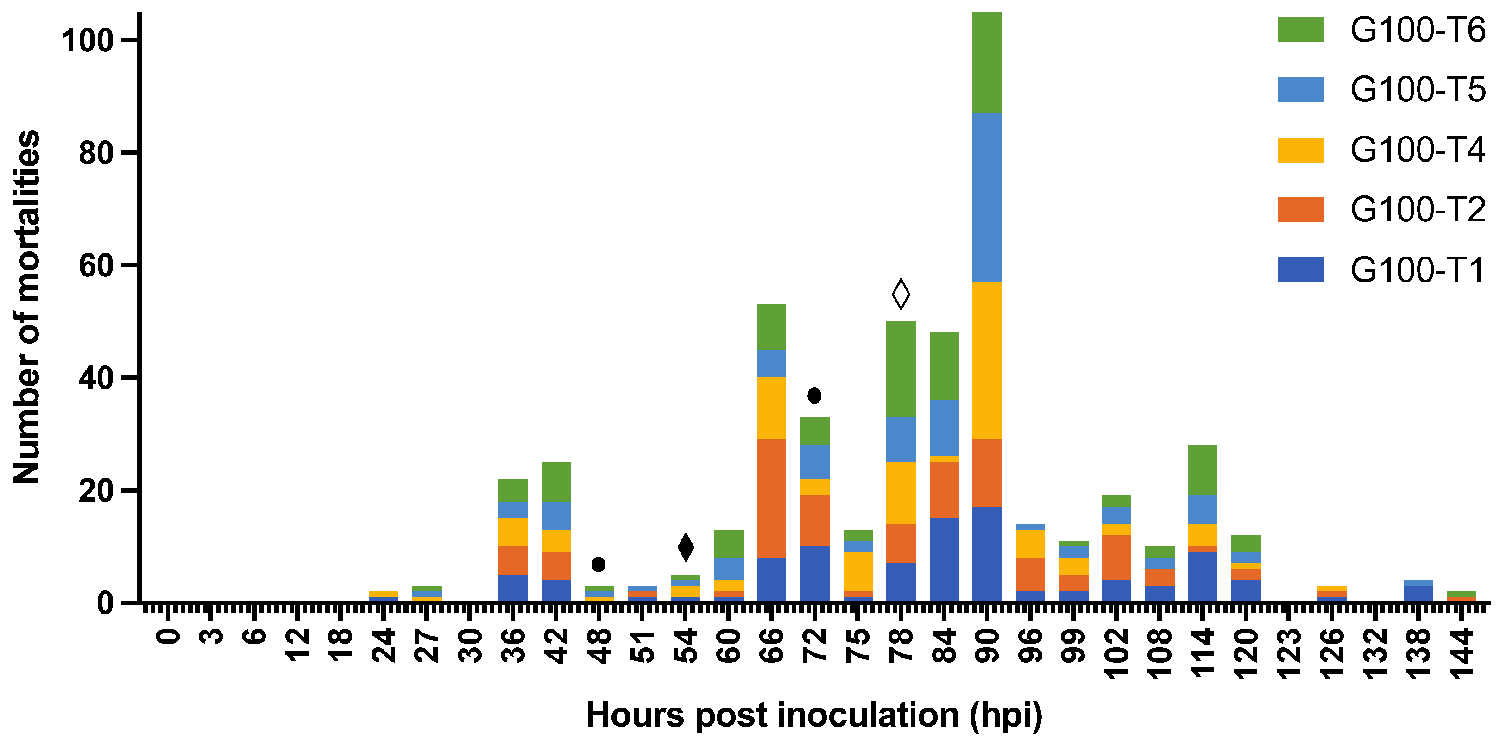
In the fourth experiment of this study, the possible role of molts, feces, and rearing water in the transmission of WSSV was investigated. These materials were collected from infected 290-liter tanks housing one hundred shrimp each that were inoculated with the second WSSV stock, which had a higher infectious titer than the first stock. This could explain the rapid spread of the disease in these infected tanks. It was demonstrated that infected rearing water collected from these tanks resulted in the highest risk of infection in sentinel populations and that WSSV transmission was water-borne.
Exposing sentinels to decanted and sieved water resulted in a slightly lower risk of infection compared to exposure to water and biofilter material. An explanation for this observation might be offered by the findings from several other studies that discovered WSSV could be detected in zooplankton and temporarily attach to phytoplankton when the virus concentration in the water was high. If plankton acted as a vector for WSSV in our experimental setup, decanting and sieving rearing water and foregoing the addition of biofilter material might have reduced this vector concentration and therefore possibly also the risk of infection.
Examining the horizontal transmission of WSSV in Pacific white shrimp
Exposing sentinels to feces produced by shrimp from infected populations did not appear to substantially facilitate WSSV transmission like the rearing water from the infected populations did. Also, exposing sentinels to molted cuticles collected from infected populations did not result in infection. Overall, the fact that cuticles and feces in relatively large quantities did not convincingly result in WSSV infection, implies that their role in WSSV transmission is potentially rather limited. It should also be considered that possible saturation of the feces by the infected tank water could not be prevented in this experimental setup. We could not rule out that this might have caused the WSD outbreak in one of the five tanks that were exposed to these feces.
Future research should focus on elucidating the transmission dynamics of WSD on a cellular and molecular level by investigating the potential sites of infection, WSSV replication, and shedding. Examining differences in the genotypes of WSD susceptible and WSD resistant lines of L. vannamei might also lead to discoveries on the molecular mechanisms that play a role during WSSV transmission.
Perspectives
The WSSV Thai-1 strain had an incubation period of 24–54 hpi, and an irreversible disease progression leading to death within 78 hpi. Shrimp infected with this strain were shedding viral DNA over the course of the disease, and this shedding reached a peak within 12 hours of the time of death. The threshold density for the occurrence of a WSD epidemic in a group infection model was 10 shrimp per 10 liters. At this density, the first cases of host-to-host transmission occurred between 30 and 48 hpi in parallel with the occurrence of the first mortalities.
Ingestion of WSSV-infected tissues did not significantly increase the number of index cases during an epidemic compared to immersion into water in which cannibalism had occurred. This could indicate that direct WSSV transmission, through the ingestion of infected tissues, plays a less important role in WSSV transmission than previously thought.
Moreover, the investigation of the role of other environmental components (water, feces, molts), showed that exposing sentinels to rearing water collected from WSSV-infected tanks resulted in a significantly higher probability of infection than exposure to feces or molts. Therefore, we postulate that the occurrence of cannibalism of infected shrimp contributes to indirect water-borne WSSV transmission by the spread of free infectious viral particles.
Now that you've reached the end of the article ...
… please consider supporting GSA’s mission to advance responsible seafood practices through education, advocacy and third-party assurances. The Advocate aims to document the evolution of responsible seafood practices and share the expansive knowledge of our vast network of contributors.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year.
Not a GSA member? Join us.
Author
-
Natasja Cox, Ph.D.
Corresponding author
IMAQUA, 9080 Lochristi, Belgium; and Laboratory of Virology, Department of Translational Physiology, Infectiology and Public Health, Faculty of Veterinary Medicine, Ghent University, 9820 Merelbeke, Belgium
Tagged With
Related Posts

Health & Welfare
Assessment of transmission risk in cooked, WSSV-infected shrimp
Exported cooked shrimp infected with White Spot Syndrome Virus (WSSV) and tested positive by PCR is considered a risk factor for the introduction of the pathogen.

Health & Welfare
A comprehensive look at the Proficiency Test for farmed shrimp
The University of Arizona Aquaculture Pathology Laboratory has carried out the Proficiency Test (PT) since 2005, with 300-plus diagnostic laboratories participating while improving their capabilities in the diagnosis of several shrimp pathogens.

Health & Welfare
Carotenoid supplements and WSSV
The effectiveness of carotenoid supplements will depend upon the severity of infection and other stress factors that affect the condition of the shrimp.
Health & Welfare
Double-stranded RNA against WSSV genes provides antiviral protection in shrimp
Silencing genes in white spot syndrome virus (WSSV) with critical roles in replication could provide a strong antiviral effect and thus reduce shrimp mortality. The authors therefore established a study to evaluate the antiviral efficacy of double-stranded (ds)RNA against non-structural WSSV genes.



