The choice of strategies to assess aquafeed ingredients can have a strong impact on the interpretation of their quality

A variety of strategies can be used to assess the nutritional quality of ingredients; however, the choice of strategies can have a strong impact on the interpretation of that information. A landmark review paper published over a decade ago has been considered the benchmark approach by which to structure research assessing the quality of ingredients. In that review, a series of five steps (and the order in which they should be done) to develop a comprehensive data set on which to base judgements about ingredient quality were proposed; 1. characterization, 2. palatability, 3. digestibility, 4. utilization and 5. functionality (processability).
Once these five steps had been achieved, a formulator could make the judicious choice as to whether to use, and with what constraints to impose, any ingredient that they were presented with. Without any one of these steps, the risk exposure substantially increased as the formulator needed to make assumptions, and this significantly increased the risk of a feed failing in one or more specifications. Typically, many studies in this domain have skipped many of these early steps and gone straight to the assessment of utilization (step 4). However, in doing so, many of these studies have ended up with erroneous outcomes and/or misleading assessments of the ingredients that they are testing, not due to any limitations of the ingredient per se, but rather failure of the researcher to observe critical formulation constraints that allow the ingredient to be assessed on a basis commensurate with its potential to supply nutrients and energy.
This article ̶ adapted and summarized from the original publication (Glencross, B.D. 2020. A feed is still only as good as its ingredients: An update on the nutritional research strategies for the optimal evaluation of ingredients for aquaculture feeds. Aquaculture Nutrition, Vol. 26, Issue 6, December 2020, Pages 1871-1883.) ̶ provides an oversight and some guidance on what steps should be undertaken to assess quality of ingredients and why those steps should be taken in that order and critically, what new steps should be considered for the assessment process in light of recent advances in the science.
Step 1: Characterization
The characterization of ingredients is an often overlooked, but critical step in the evaluation process. As such, this initial step in the ingredient evaluation process remains as important as ever. For formulators to make use of technical documentation on ingredients, the users of that information must be able to relate the data to a particular type of ingredient. However, there is substantial variability in both the composition and nutritional values (as defined by the digestibility of nutrients from the ingredient) of most ingredients. Details on the species, origins, processing and/or storage history, let alone a chemical characterization, are often absent in a much of the scientific literature. Any chemical characterization needs to include, as a minimum, the basic parameters used to formulate feeds and/or allow clear assessment of the ingredient.
Clearly for other types of ingredients, such as plant-derived materials, there would also be some merit in including data on parameters such as starch, non-starch polysaccharides, acid-detergent and neutral detergent fiber and lignin. The methods for analysis should follow standardized methods such as those recommended by analytical associations such as AOAC International and others.
Without some form of characterization, the value of the work is somewhat diminished as it becomes difficult for the reader/user of the data to effectively relate the work to their materials. By providing a comprehensive characterization, it becomes much easier to relate the assessment to other materials and/or obtain the same material. Another element to characterization that is gaining importance, and over time may warrant its own step, is an assessment of the sustainability of an ingredient. Various strategies have been examined to define sustainability of ingredients, but life cycle assessment is perhaps the approach gaining most favor.
Step 2: Palatability
Before the impact of the nutrients within a feed can be measured on animal performance, the animal clearly must ingest that feed. A decision hierarchy framework has been suggested as a means of defining the nature of the responses to feeds to help define how the feed is specifically impacting the response. Fig. 1 shows how the influence of a feed (and by inference its ingredients) on an animal’s response can be assessed to define the specific aspect of palatability that is affected (and note that this response might be positive or negative). It is clear that any factor(s) that negatively affect this hierarchy are going to limit the potential of the ingredient. Thus, one of the qualities of any ingredient that is critically important to a feed is its effect on palatability of the feed. Obviously, if an ingredient reduces feed intake due to negative effects on palatability, it has some limitations as a potential feed ingredient.
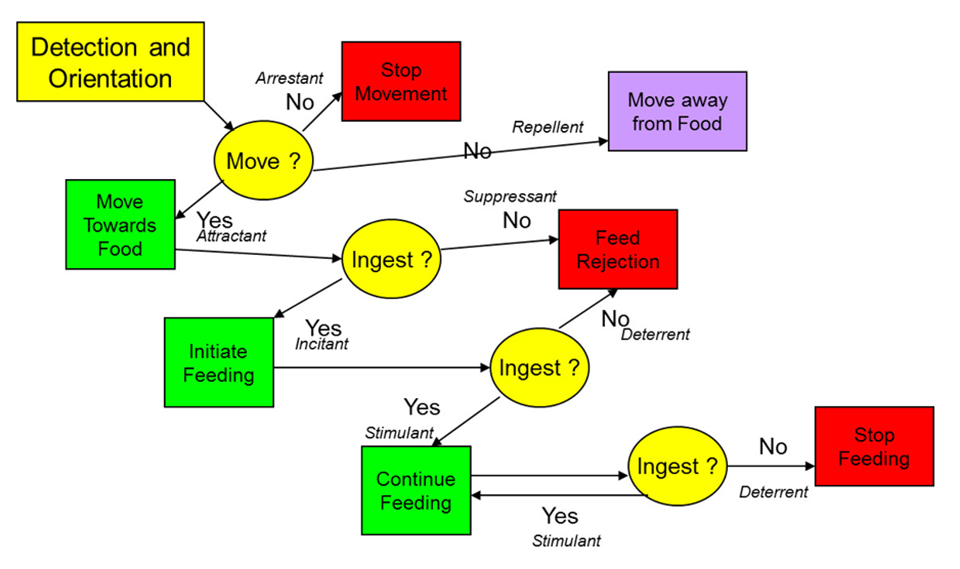
Conversely, those ingredients that can stimulate intake, and thereby improve palatability, have added value as ingredients. Some studies show how this variation in palatability can affect greater than 80 percent of the variability in growth response to diets testing alternative ingredients. In studies where fish are fed a fixed ration, it becomes impossible to assess the impact of diet (and by extension the test ingredient) on palatability responses. It should also be noted that effects on palatability of diets can often be detected within days of introduction and are usually at their most sensitive point of assessment within the first 10 days (minus days 1 to 3) of an animal being fed that new diet. After this period, the animal may begin to adapt to the diet and the ability to discriminate palatability effects accordingly becomes reduced.
Step 3: Digestibility
The assessment of the digestibility of nutrients and energy from diets and ingredients provides one of the clearest ways of unambiguously defining the nutritional value of an ingredient to an animal. By measuring the relative disappearance of nutrients between the feed and feces, those nutrients available to an animal can be effectively measured. It is these nutrients that are used to drive feed intake (in response to digestible energy demands) and underpin growth. Therefore, variability in this digestibility is one of the critical points of variability in feed quality.
There are a range of methods that can be used to assess diet digestibility, and these can all potentially influence the absolute values determined in any given assessment. The important element to the reliable and consistent assessment of digestibility is to minimize non‐nutritional effects (i.e. minimize effects of environment or sampling method). For a comprehensive review of the range of strategies and their limitations, see https://doi.org/10.1111/j.1365-2095.2007.00450.x .
Step 4: Utilization
The assessment of nutrient and/or energy utilization is mostly commonly undertaken by a growth/feeding trial approach. In this strategy, a diet (or series of diets) is fed to the target species and the phenomic [systematic study of phenotypes, the composite observable characteristics or traits of an organism] responses typically assessed. While primarily responses such as feed intake and weight gain are the main points of assessment, many other nutritional responses can also be measured. For a more comprehensive assessment of the vagaries of different growth trial strategies, see https://doi.org/10.1111/j.1365-2095.2007.00450.x .
While these trials are the most commonly encountered among the literature, their outcomes are largely predictable subject to diet specification choices, digestibility of ingredients used and the palatability of the diets produced. In those situations where the diets are formulated to the same specifications and on a digestible nutrient basis, the growth responses that result from the feeding study are usually largely just a reflection of feed intake (palatability) variation.
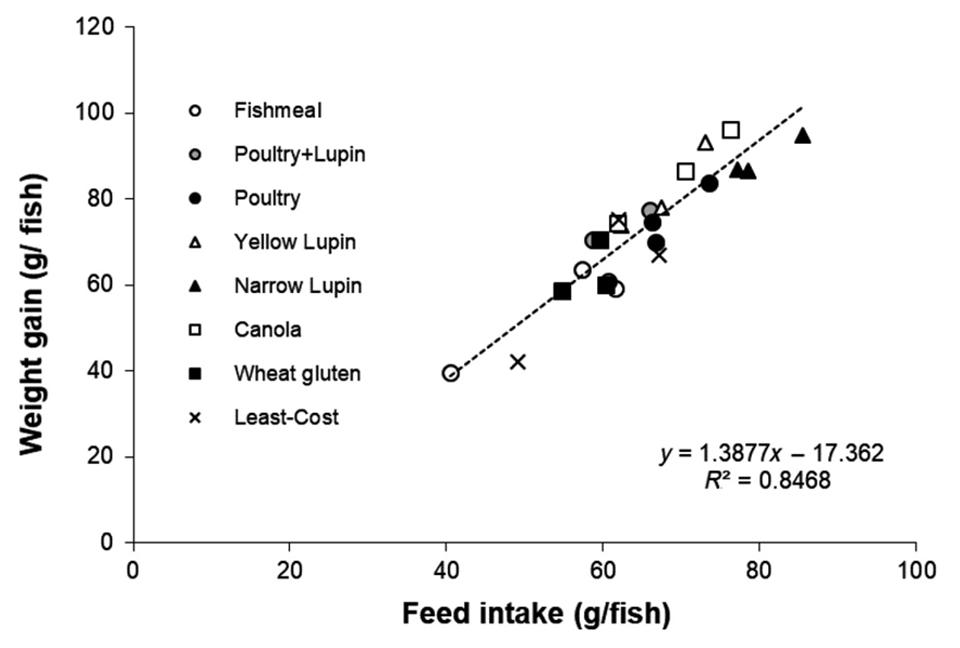
Fig. 2 shows a typical such response from the evaluation of a suite of alternative ingredients fed to Asian sea bass (Lates calcarifer), where the feed intake alone accounts for over 80 percent of the variation in the growth response. Occasionally, unexpected responses do occur, and this is arguably where the true value of the utilization study comes into effect. It can also be argued that such studies allow for subtle effects to amortize over time and then these minor variables can be then be observed more clearly. Certainly, such trials are critical for determination of nutrient requirement and metabolic response studies but are arguably less valuable for ingredient evaluation studies.
The formulation strategy used to develop test diets can have a strong bearing on the interpretation of the assessment of ingredients. While most commercial diets are now days formulated on a digestible protein and energy basis, a majority of scientific literature still presents diets formulated on a crude basis. The reason why this is problematic can be demonstrated via the hypothetical example in Fig. 3. In this example, we consider two diet formulation strategies, one formulated solely to crude nutrient specifications and the other to a digestible nutrient specifications. In this example, we can see the impact of the inclusion of an alternative ingredient containing 650 grams per kg protein, with a digestibility of 75 percent, added to each series of diets at 100 grams per kg increments, with the remaining diet protein 85 percent digestible.
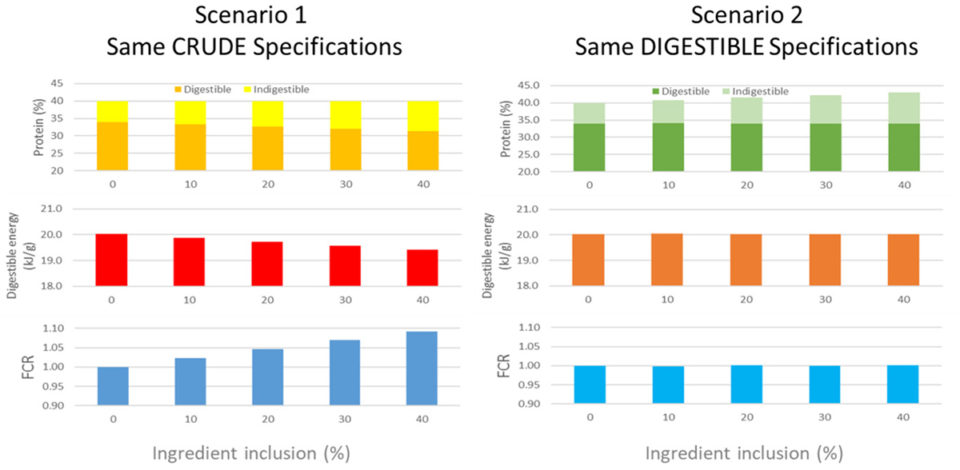
From this model, we can see that in the crude specification scenario that the level of digestible protein in the diet declines from 340 grams per kg, with no alternative inclusion, to about 310 grams per kg when the alternative is included at 400 grams per kg. By contrast in the digestible specification scenario, the digestible protein level stays constant at 340 grams per kg, while the crude protein level increases from 400 grams per kg to about 430 grams per kg.
These differences in digestible protein levels in each series of diets would have direct impact on the digestible energy density in each scenario as well. In response to these differences, if we were to feed such diets to a species like Atlantic salmon, we would see a clear difference in feed conversion based on the fact that such species are clear responders to dietary energy density, typically responding with a 0.15 decline in FCR for every MJ increase in digestible energy content in a diet. Accordingly, we would then see quite different feed conversion ratio (FCR) responses in each scenario. In the crude specification scenario, it would be reasonable to suggest that the alternative ingredient causes a decline in performance at all inclusion levels and therefore should be considered a low-grade ingredient.
By contrast, the digestible specification scenario shows no response in performance to the use of the alternative, and therefore, it would be reasonable to suggest that the alternative ingredient is well utilized and tolerated at all inclusion levels and therefore should be considered a high-grade ingredient. Of course, between each scenario the ingredient is the same, only the formulation strategy has changed.
When diets are formulated to non-constraining specifications (i.e. specifications where no specific nutrients are limiting or close to limitation levels), as like occurs in typical commercial diet specifications, using this approach all too often results in a simple null-hypothesis outcome with no differences observed among the diets. Results such as this provide little useful information in terms of application of the ingredients being assessed due to the large margins for error built into the diet formulations to specifically limit such responses being observed.
While the relevance of a commercial style specification might be argued, the lack of sensitivity in using such a specification also needs to be considered. Sometimes a commercial analogy might not be the most appropriate strategy to answer a nutritional question.
Step 5: Immunological and health allied assessments
A growing priority in the assessment of feeds and ingredients in animal diets is their impact on the immune response and general robustness (health) of the animal. There are a variety of strategies that are being used to examine these parameters, including specific pathogen (disease) challenges, which can be undertaken to assess the impacts of diets on the immunogenic function of fish against a particular pathogen of interest. In such studies, the animals are typically fed the diets and then challenged with a pathogen (usually a virus or bacterium) and the survival and immune responses of the population subsequently assessed.
Some common cellular and biochemical assessments include a histological analysis to look at tissue-specific damage arising from the use of any particular ingredient. Biochemical tests include those such as measuring lysozyme or superoxide dismutase activity.
Now that you've reached the end of the article ...
… please consider supporting GSA’s mission to advance responsible seafood practices through education, advocacy and third-party assurances. The Advocate aims to document the evolution of responsible seafood practices and share the expansive knowledge of our vast network of contributors.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year.
Not a GSA member? Join us.
Author
-
Brett D. Glencross, Ph.D.
Institute of Aquaculture
University of Stirling
Stirling, UK[107,117,46,99,97,46,114,105,116,115,64,115,115,111,114,99,110,101,108,103,46,100,46,98]
Tagged With
Related Posts

Health & Welfare
Advances in tilapia nutrition, part 2
Nutrition has an important role on growth, performance and flesh quality of tilapia. Part two of this two-part series looks into mineral supplementation and feeding strategy.

Health & Welfare
Cathepsin enzymes, part 1
Cathepsins B, D and L are considered critical in fish muscle post-mortem modifications or gel softening during the setting of surimi.

Intelligence
Cost to benefit, fish skin yields unique gelatin products
Fish skin can be processed into food-grade gelatin. Unlike gelatins made from cattle and pig skins, those from coldwater fish remain liquid when not refrigerated.

Health & Welfare
Scotland study evaluates immune responses, disease resistance in clonal lines of Nile tilapia
Once a pathogen breaches the physical barriers of its host, it encounters the nonspecific immune response components that attack and help destroy the pathogen.


