It’s important to maintain desirable acidity levels in aquaculture ponds

Farmers are aware of the importance of monitoring levels of dissolved oxygen in their production systems and of providing supplemental aeration in intensive fish and shrimp ponds. However, other water quality parameters – as simple as they may look – are often overlooked. One such parameter is pH, which in chemistry refers to a numeric scale used to specify the acid or alkaline condition of an aqueous solutions.
In a practical sense, water pH may range from 0 to 14 and is related to the concentration of hydrogen ion (a strong acid) in pond water. Pond water may be acid (pH < 7.0), neutral (pH= 7.0) or alkaline (pH > 7.0). In general, farmed fish and shrimp exhibit better production performance and health at water pH values ranging from 7.5 to 8.5, as these values match the pH of their blood and hemolymph (Fig. 1).
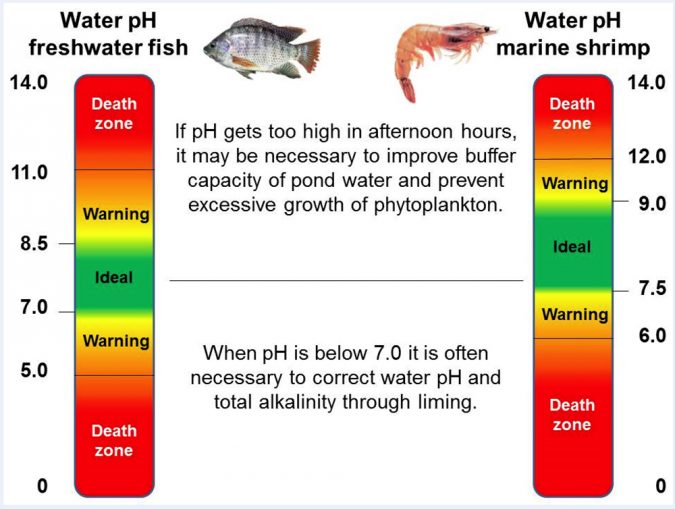
Nonetheless, as with dissolved oxygen, water pH values can experience wide fluctuations during the day in green water ponds (Fig. 2). Values of pH near or above 9.0 are quite common during afternoon hours, due to the intense photosynthesis activity by microalgae (phytoplankton), even in ponds with well buffered waters (i.e., waters that are rich in carbonates and bicarbonates and, thus, of high total alkalinity titration and capable of maintaining more stable pH values). Extreme pH, by itself, depresses feeding activity, reduces growth, negatively affects FCR and suppresses the immune response of cultured fish and shrimp.

Water pH determines ammonia toxicity to fish and shrimp
Water pH modulates the risk of ammonia toxicity. Ammonia is a toxic compound often present in pond water, a metabolic waste excreted mainly through the gills of fish and shrimp, and it is also generated via the microbial degradation of organic matter (decaying microalgae, feces, organic fertilizers and unconsumed aquafeed). Some inorganic fertilizers used in aquaculture may also be sources of ammonia. Total ammonia concentration in pond water can be easily measured using a reliable, commercially available water quality test kit. Ammonia comprises NH4+ (the ammonium ion, the less toxic form) and NH3 (the unionized, gaseous, and more toxic – to aquatic animals – form of ammonia). Total ammonia in intensively managed fish and shrimp ponds may vary from nearly zero to as high as 8 to 12 ppm.
Many factors may influence the total ammonia concentration in an aquaculture pond. The most important ones are feeding rate, feed quality and rate of water exchange. Water pH determines the percentage of each ammonia form in pond water. At low pH, the less toxic form NH4+ predominates (Table 1). However, as pH increases, NH4+ will shift to the toxic form and increasing the concentration of NH3 in the water. In freshwater, at pH 7.0, only 0.7 percent of the total ammonia will be in the toxic form of NH3, while at pH 9.0 this percentage increases to about 42 percent, and further to 88 percent at pH 10 (Table 1).
Therefore, a freshwater pond with 6 ppm total ammonia and water pH 7.0 (0.7 percent NH3) will have only 0.042 ppm of the toxic form of ammonia (6 ppm x 0.7/100 = 0.042 ppm NH3). However, in green water ponds, pH may shift to 9.0 or above during afternoon hours. At pH 9.0 (42 percent NH3), a pond with 6 ppm total ammonia will have 2.5 ppm of NH3 (6 ppm x 42/100 = 2.5 ppm NH3).
| Water pH | Percentage (%) of taxi ammonia (NH3) on the total ammonia at 28 degrees-C* | Maximum total ammonia (ppm or mg/L) to not exceed the 0.2 ppm of NH3 | ||
|---|---|---|---|---|
| FW (0 ppt) | SW (36 ppt) | FW (0 ppt salt) | SW (36 ppt salt) | |
| 6.5 | 0.22 | 0.18 | 89.1 | 108.7 |
| 7.0 | 0.71 | 0.58 | 28.3 | 34.5 |
| 7.5 | 2.20 | 1.81 | 9.1 | 11.1 |
| 8.0 | 6.64 | 5.51 | 3.0 | 3.6 |
| 8.5 | 18.36 | 15.57 | 1.1 | 1.3 |
| 9.0 | 41.67 | 36.83 | 0.48 | 0.54 |
| 9.5 | 69.23 | 64.84 | 0.29 | 0.31 |
| 10.0 | 87.67 | 85.36 | 0.23 | 0.23 |
Toxic ammonia levels below 0.2 ppm generally cause no harm to farmed animals. However, values above 1 ppm are in the stressful and lethal range (0.7 to 3 ppm of NH3) for many fish and shrimp species. Therefore, the 0.2 ppm NH3 should be adopted as a “warning level” and changes in the management practices must be implemented to prevent NH3 from significantly exceeding such value. At a pH of 9.0, the 0.2 ppm NH3 warning level will be reached at a total ammonia concentration of 0.5 ppm. At pH 7.0, the warning level will only occur at a total ammonia concentration of 28 ppm (34 ppm in salt water), which is a very unlikely concentration in standard managed fish and shrimp ponds. However, some particular fish and shrimp species may be more sensitive to ammonia and farmers may have to adopt a lower warning level.
Fortunately, high pH values that can potentiate ammonia toxicity to fish and shrimp occur mainly during noon and afternoon hours. At night, with the cessation of photosynthesis and the increase in the concentration of carbon dioxide (CO2), the water pH decreases. The night-time drop in pH provides the fish and shrimp with relief from the afternoon ammonia toxicity. Nonetheless, on the next day the pH will rise and animals will be exposed again to toxic concentrations of ammonia. Many fish and shrimp farmers attempting to maximize production by increasing stocking, feeding and aeration end up experiencing poor production results. Fish and shrimp repeatedly exposed to toxic levels of ammonia will present reduced feed intake, poor growth, inefficient FCR and depressed immunity.
Severe disease outbreaks, high mortality and increased production costs can be consequences of frequent exposure of fish and shrimp to high afternoon water pH with toxic concentrations of ammonia. Therefore, farmers should not overlook the importance of monitoring pH and total ammonia in their ponds.
High pH and ammonia auto-intoxication
Fish and shrimp catabolize any surplus dietary protein (amino acids) to render energy, glucose and glycerol (a fat precursor). The end product of such catabolism is ammonia, and it needs to be rapidly excreted from the blood to the water. In general, the higher the protein (amino acids) content in the feed, the more ammonia fish and shrimp will generate through the catabolism of amino acids. Ammonia excretion by simple diffusion of NH3 from blood to water is easily accomplished when the water pH is lower than the blood pH (the blood pH of freshwater fishes ranges from 7.4 to 7.8).
However, when the water pH is much higher than the blood pH, ammonia diffusion through the gills is impaired and ammonia will build up in the fish blood, resulting in a condition known as “ammonia auto-intoxication” (Fig. 3). Many species of fish experience increased levels of ammonia in their blood when exposed to water pH above 9.0. Ammonia auto-intoxication, though quite common in fish, has not yet been reported in shrimp. Nonetheless, as the mechanisms of ammonia excretion in fish and shrimp are quite similar, it is also possible that shrimp can accumulate ammonia in their hemolymph when exposed to high pH, and we recommend further research to address this hypothesis.

In fish, ammonia auto-intoxication may occur independently of the presence of toxic ammonia in water. Fish can build up sub-lethal or even lethal levels of ammonia in their blood after a sequence of days or weeks exposed to pH 9.0 or higher, a common condition in green water ponds during bright sunshine days. Massive fish losses due to ammonia auto-intoxication are quite common in heavily-fertilized/high protein-fed hatchery ponds. In these ponds, phytoplankton levels become excessive and cause water pH levels to rise above 9.5 during the afternoon hours.
As hatchery ponds are often shallow and have respiration rates well below the photosynthesis rate, the high pH condition persists through most of the night, not providing fish with much relief or opportunity to resume normal ammonia excretion. Ammonia auto-intoxication is further aggravated by excessive feeding and/or the use of high protein feeds, a common practice in many fish hatcheries. Farmers seldom relate such mortalities to ammonia toxicity, as ammonia is practically absent from pond water due to its fast removal by microalgae that use it as a source of nitrogen. Ammonia readings using a test kit often show zero total ammonia in the water. However, endogenous ammonia builds up in fish blood, causing distress and mortality.
Although less common, ammonia auto-intoxication of fish may also occur in growout ponds. As these ponds are deeper than hatchery ponds, fish may find some relief moving to deeper pond strata if dissolved oxygen levels there are adequate. The deeper pond water generally has lower pH levels compared to surface waters concentrated in microalgae. Thus, this high pH avoidance behavior may explain the reduced feeding activity often observed in growout ponds during the afternoon hours, even under adequate oxygen and temperature, and “zero” toxic ammonia in the surface water.
Fish start to alternate afternoons of good and bad feeding responses, reducing their growth potential and impairing feed conversion. Therefore, sub-lethal ammonia auto-intoxication acts silently, depressing growth and feed efficiency (FCR), with a significant impact on production cost and farmers’ profitability. Animals will also become immunosuppressed and more susceptible to diseases. Chronic mortality due to bacterial infections should be expected. Blood ammonia may reach lethal levels, leading to sudden and massive fish kills in aquaculture ponds.
Significant economic losses are likely to result due to delayed growth, longer production cycles, worsened FCR, increased incidence of bacterial diseases and eventual sudden and massive death due to ammonia intoxication if the farmers do not pay attention to water pH and ammonia in their ponds.
Fish intoxicated with ammonia show neurotoxic disturbances, erratic or whirling swimming, muscular spams, increased gill ventilation and gasping at water surface, as if showing signs of asphyxia, even at adequate oxygen levels (hyperplasia of gill lamellae is behind this breathing difficulty). Intoxicated fish often look for water inlets, or will be stationary in shadowed areas or underneath plants on the pond edges, probably searching for spots with somewhat lower pH than that in open pond water. Fish will prefer to stay at the lower strata if oxygen is available, finding a water layer of lower pH in an attempt to relieve ammonia auto-intoxication. It is quite common to find dead fish with gills covered with mud, probably from a last attempt to avoid death.
After a sudden, massive fish kill in growout ponds, it is common to observe most of the dead fish in the deepest area, because just before their death they were struggling to survive at the deepest strata. Checking ammonia levels in the blood of moribund or freshly dead fish will help to determine if mortality was caused by ammonia intoxication.
Many farmers often relate sudden and unexpected massive fish kills to a “toxic algae” bloom, as they notice that intoxicated fish swim erratically and are unable to direct themselves towards the pond aerators, as normal fish do in a condition of low oxygen. Ammonia auto-intoxicated fish also behave the same way, and many episodes of massive fish kills thought to be caused by “toxic algae” are more likely due to ammonia intoxication or auto-intoxication.
Preventative actions
Aquafarmers should not overlook the importance of monitoring pH and total ammonia in their ponds. Weekly afternoon water pH and ammonia analyses should be performed in static ponds receiving over 100 kg of feed/ha/day. Farmers must act when pH is becoming too high and/or toxic ammonia levels are approaching 0.2 ppm, the “warning level” suggested in this article.
Reducing the feeding rate (to reduce the input of ammonia and nutrients), increasing aeration and water circulation (to speed up ammonia oxidation) and partial water exchange (to dilute ammonia and other nutrients, while simultaneously thinning out phytoplankton) are the first management practices that should be considered. Hatchery managers should avoid excessive fertilization of hatchery ponds, overfeeding of fish and the use of starter diets with protein levels that are too high. A more frequent monitoring of afternoon water pH and total ammonia levels will help to anticipate any pH/ammonia issues that may lead to fish mortality.
As pH has a profound impact on the concentration of toxic ammonia in a pond, the most effective way to lessen the impact of NH3 on health and performance of fish and shrimp is to prevent spikes in water pH. Thus, the control of phytoplankton and the increase of the buffering capacity of pond water (through liming, when necessary) are important management practices to prevent ammonia toxicity. Farmers should check heavily fed, green water ponds for water pH in the afternoon (15:00 to 16:00 h) on bright sunshine days and on the following morning (7:00 h). If pH variation in a pond exceeds two or more units, it means either that water buffering capacity is too low, or that phytoplankton is too excessive (intense photosynthesis and respiration).
Total alkalinity must be further checked and the pond should be limed if values are below 30 mg/L equivalent CaCO3. If total alkalinity seems adequate (above 30 mg/l), farmers should start to control the phytoplankton. Reducing feeding rates, partial water exchange, physical removal of surface algae blooms/scum, use of algaecides, nutrient manipulation, increase water turbidity with clays or dies, circulation of pond water through wetlands, biological control by filter feeding fish, zooplankton, and/or mollusks, shading and nutrient competition by aquatic macrophytes, and other measures are some of the tools farmers may consider to use to fight excessive phytoplankton concentrations.
Final remarks
Looking to maximize production, fish and shrimp farmers often increase aeration, feeding and stocking rates. Such conditions will likely increase problems related to high pH and ammonia toxicity, mainly in ponds where water exchange is limited. Significant economic losses are likely to result due to delayed growth, longer production cycles, worsened FCR, increased incidence of bacterial diseases and eventual sudden and massive death due to ammonia intoxication if the farmers do not pay attention to water pH and ammonia in their ponds.
In many cases, limiting the standing crop and feeding rate to safe levels will improve water quality, growth, FCR, and survival, reducing production cost and increasing farm profitability. Such attention to the interconnectedness of pH and ammonia poisoning can reduce production risks and improve the business sustainability of many fish and shrimp farms.
Now that you've reached the end of the article ...
… please consider supporting GSA’s mission to advance responsible seafood practices through education, advocacy and third-party assurances. The Advocate aims to document the evolution of responsible seafood practices and share the expansive knowledge of our vast network of contributors.
By becoming a Global Seafood Alliance member, you’re ensuring that all of the pre-competitive work we do through member benefits, resources and events can continue. Individual membership costs just $50 a year.
Not a GSA member? Join us.
Author
-

Fernando Kubitza, Ph.D.
Acqua Imagem Serviços Ltda.
Rua Evangelina Soares de Camargo
115 Jardim Estádio – Jundiai/SP – CEP 13203-560 Brazil
Tagged With
Related Posts

Intelligence
Brazilian aquaculture: Constraints and challenges (Part 1)
The Brazilian aquaculture industry has been growing steadily during the last two decades. Despite facing a number of challenges it is looking at continued growth and a larger role in the export markets.

Intelligence
Brazilian aquaculture: Constraints and challenges (Part 2)
Brazil will have to deal with an adverse economic and political environment in the next few years. One should expect high value fish products like shrimp, tilapia, Chilean salmon and cod being replaced by more affordable seafood and alternative meats, as consumers keep losing purchasing power due to inflation, unemployment and monetary devaluation.

Health & Welfare
Common salt a useful tool in aquaculture, part 1
The preventive use of common salt (sodium chloride) by commercial producers of freshwater fishes has many benefits, including helping with the routine prevention of losses due to diseases, stress and mishandling during transport, harvesting, grading, counting, weighing and induced spawning.

Health & Welfare
Common salt a useful tool in aquaculture, part 2
The preventive use of common salt (sodium chloride) by commercial producers of freshwater fishes has many benefits, including helping with the routine prevention of losses due to diseases, stress and mishandling during transport, harvesting, grading, counting, weighing and induced spawning.

